Believe it or not, every white mouse is unique in its own way! Despite seeming identical to the untrained eye, each mouse carries its own distinct features. Just like humans, each mouse exhibits unique characteristics and behaviors, and reacts differently to various stimuli, drugs, or treatments. This makes individual identification and tracking an essential requirement for scientific research, which often necessitates individualized follow-ups of all utilized animals.
Historically, a variety of methods have been used to distinguish between individual mice in a laboratory setting (Donovan & Brown, 2006). Here we present a non-exhaustive list of identification methods. Note that some of these methods reflect past attitudes towards animal welfare, which have since evolved to recognize mice as sentient beings capable of experiencing both positive and negative emotions (Poole, 1997).
Identification methods can be permanent or temporary; the choice of one or the other is made based on the scientific topic studied. One temporary and non-invasive method is marking the tail with a marker pen. This has been used for short-term identification. It is easy to perform, non-invasive, but due to the mice’s regular grooming habits, such markers tend to wear off, requiring frequent reapplication.
For studies requiring longer durations, more permanent methods of identification have been implemented, although some of these techniques can be invasive and cause varying degrees of discomfort or stress for the animal (Kasanen et al., 2011; Leslie et al., 2010). These methods, including toe clipping and ear notching, involve the removal of a part of the animal’s body.
Toe clipping or ear notching consists of cutting toes or ears with a specific code to identify each mouse individually. One of them code is shown in Figure 1.
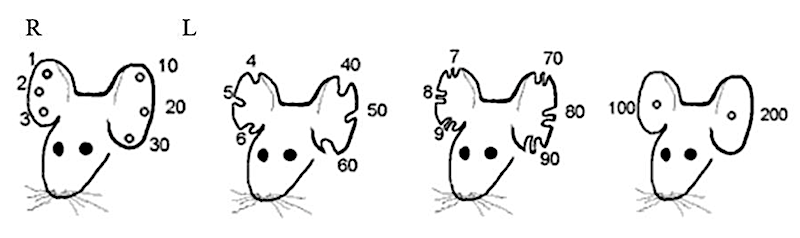
Figure 1: Ear Notching for Identification in Mice | Animals in Science (queensu.ca)
Even though these invasive methods were often performed under anesthesia, they were carried out most of the time without adequate pain management, and long-lasting discomfort can persist, particularly with toe clipping. As our understanding and attitudes toward animal welfare have evolved, there’s been a strong move away from such methods (Committee for the Update of the Guide for the Care and Use of Laboratory Animals et al., 2011; Norecopa, 2008). These methods are now strictly regulated and limited to specific conditions by French legislation (Mormede et al., 2019).
Alternative methods such as tattooing and ear tagging, although less invasive, may still present challenges due to the small size of the mice, potentially causing discomfort and requiring meticulous care in application (Roughan & Sevenoaks, 2019). The weight of the mice (often less than 15g at the time of identification) may complicate both methods and experimenters should be well-trained and careful to avoid skin damage. These skin damages may create infections, discomfort, and/or pain for the animal (Roughan & Sevenoaks, 2019). To refine existing methods, the use of local analgesia is sometimes discussed, in the context of tattooing, for example (Keating et al., 2012).
An increasingly favored method for animal identification is the use of subcutaneous chips, similar to those used in household pets. This method, although it requires the placement of a chip (about 1.5 mm in diameter and 8.5 mm in length) under the skin, tends to cause minimal discomfort after the healing process and offers a reliable way of identification. The identification method can be further improved by anesthetizing the animals to avoid pain during injection.
Here at TransCure bioServices, we’ve chosen to utilize this latter method. After a brief acclimation period following their arrival, each mouse is implanted with a subcutaneous chip, administered under anesthesia to minimize discomfort. The microchip contains an unforgeable digital code linked to our animal research software. This unique ID number follows the mouse throughout the full humanization process and the client study. It is now even easier for our collaborators to identify a mouse, deliver the proper dosing, and record clinical symptoms. The microchip is scanned with a digital reader, which reduces the number of times and the duration an animal needs to be handled. Microchip identification is unarguably a welfare improvement. It is a compelling process and a true accomplishment in line with our AAALAC accreditation.
Other identification methods are currently being developed with varied success. For example, Cameron and collaborators have developed a biometric noninvasive individual identification method that uses the rodent’s ear blood vessel pattern (see Figure 2). Other methods in development include a micro-chip “p-Chip” for ear or tail implantation (Pharmaseq, 2011); luminescent micro-tattoo LMT (Dahlborn et al., 2013).
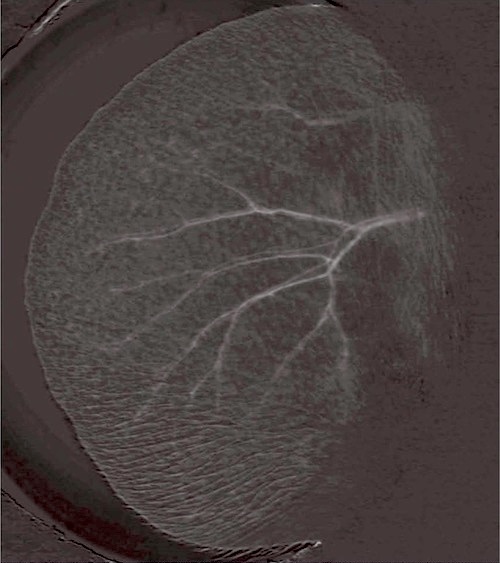
Figure 2: Image of an ear blood vessel pattern of mice, for biometric identification (Cameron et al., 2007).
Ultimately, while our methods of identification have evolved considerably over the past decades, our commitment to improving animal welfare has remained steadfast. TransCure bioServices is dedicated to advancing our research practices and ensuring the highest standards of respect and care for the animals that play such a vital role in scientific advancement.
References:
Cameron, J., Jacobson, C., Nilsson, K., & Rögnvaldsson, T. (2007). A biometric approach to laboratory rodent identification. Lab Animal, 36(3). www.labanimal.com
Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and LifeStudies, & National Research Council. (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. In National Research Council 2011 (Ed.), Guide for the Care and Use of Laboratory Animals (8th ed.). National Academies Press. https://doi.org/10.17226/12910
Dahlborn, K., Bugnon, P., Nevalainen, T., Raspa, M., Verbost, P., & Spangenberg, E. (2013). Report of the federation of european laboratory animal science associations working group on animal identification. Laboratory Animals, 47(1), 2–11. https://doi.org/10.1177/002367712473290
Donovan, J., & Brown, P. (2006). Animal Identification. In Care and Handling of Laboratory Animals.
Kasanen, I. H. E., Voipio, H. M., Leskinen, H., Luodonpää, M., & Nevalainen, T. O. (2011). Comparison of ear tattoo, ear notching and microtattoo in rats undergoing cardiovascular telemetry. Laboratory Animals, 45(3), 154–159. https://doi.org/10.1258/la.2011.010113
Keating, S. C. J., Thomas, A. A., Flecknell, P. A., & Leach, M. C. (2012). Evaluation of EMLA Cream for Preventing Pain during Tattooing of Rabbits: Changes in Physiological, Behavioural and Facial Expression Responses. PLoS ONE, 7(9). https://doi.org/10.1371/journal.pone.0044437
Leslie, E., Hernández-Jover, M., Newman, R., & Holyoake, P. (2010). Assessment of acute pain experienced by piglets from ear tagging, ear notching and intraperitoneal injectable transponders. Applied Animal Behaviour Science, 127(3–4), 86–95. https://doi.org/10.1016/j.applanim.2010.09.006
Mormede, P., Guy, N., Médale, F., Nivet-Antoine, V., Tarpin, M., Gonin, P., Picavet, S., Mary, V., Duclaux, S., Ghaleh, B., Behar-Cohen, F., Dudoignon, N., Desfontis, J.-C., Picavet, E., Bourg, D., Desmoulin-Canselier, S., Raphaël Larrère, G., Bonnet, S., Nouët, J.-C., … Briard, L. (2019). Recommandation sur la technique d’amputation de phalange comme méthode d’identification et de caractérisation génétique chez les rongeurs. https://www.syrcle.network/
Norecopa. (2008). Toe clipping in mice: an evaluation of the method and alternatives. norecopa.no
Pharmaseq. (2011). Tagging of Laboratory Mice Using Electronic p-Chips. www.pharmaseq.com
Poole, T. (1997). Happy animals make good science. Laboratory Animals, 31, 116–124.
Roughan, J. V., & Sevenoaks, T. (2019). Welfare and scientific considerations of tattooing and ear tagging for mouse identification. Journal of the American Association for Laboratory Animal Science, 58(2), 142–153. https://doi.org/10.30802/AALAS-JAALAS-18-000057