PDX Humanized Mice: Patient-derived xenograft models for translational research in oncology
Humanized mouse models engrafted with patient-derived xenografts (PDX) offer a promising approach for oncology research, providing a better understanding of human tumors and facilitating the development of personalized treatments.
Champions Oncology and TransCure bioServices partnership
 Champions Oncology have developped more than 1,500 different tumor models from different patients in the primary and/or metastatic phase. PDX models have been generated using patient-derived samples from various types of cancer, including colorectal cancer, lung cancer, liver cancer, pancreatic cancer, melanoma, carcinoma, sarcoma, and other malignancies.
Champions Oncology have developped more than 1,500 different tumor models from different patients in the primary and/or metastatic phase. PDX models have been generated using patient-derived samples from various types of cancer, including colorectal cancer, lung cancer, liver cancer, pancreatic cancer, melanoma, carcinoma, sarcoma, and other malignancies.
Humanized mice model engrafted with Patient-Derived Xenografts (PDX) : Exploring Advantages and Limitations of the Model in Translational Cancer Research
The engraftment of patient-derived tumor fragments into humanized mice, known as PDX models, allows for the presence of human tumor tissue, stromal elements and a complete human immune system in CD34+ humanized mice.
PDX humanized mouse models offer several advantages, including the representation of the patient’s tumor microenvironment (TME) and its diversity, the potential for developing personalized therapies, the identification of therapeutic targets and the interaction with a complete human immune system.
However, PDX mouse models have certain limitations. Establishing and maintaining PDX mouse models can be time-consuming and technically challenging compared to humanized mice engraft with cell-derived xenograft (CDX). Furthermore, PDX mouse models are prone to alterations during passaging, potentially affecting their representativeness over time.
Tumor Engraftment and Protocol in Humanized Mice for Preclinical Research
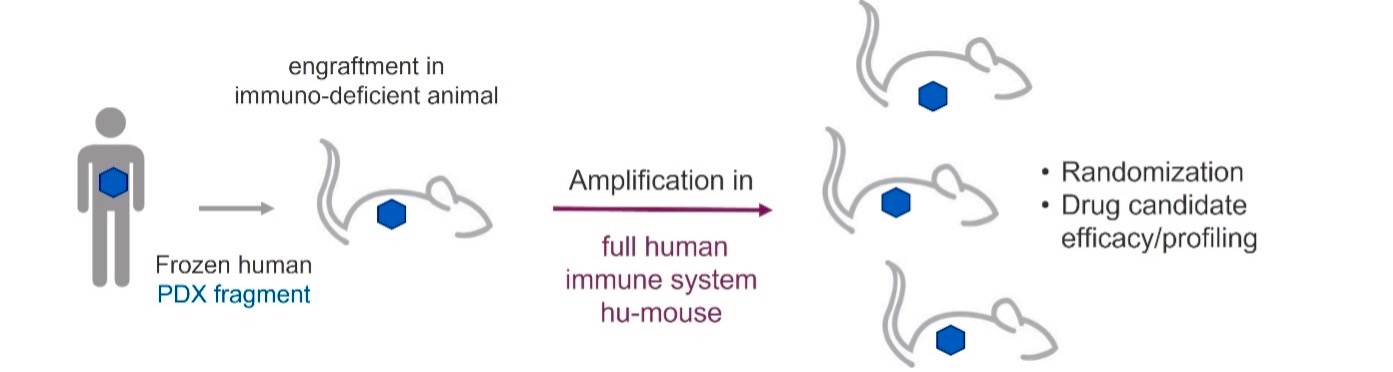
Patient-derived xenograft (PDX) samples are engrafted subcutaneously into the CD34+ humanized mice. CD34+ humanized mice offer a fully functional human immune system including T and B lymphocytes, macrophages, DC and NK. The hydrodynamic customizable gene delivery promotes proliferation of different human immune population.
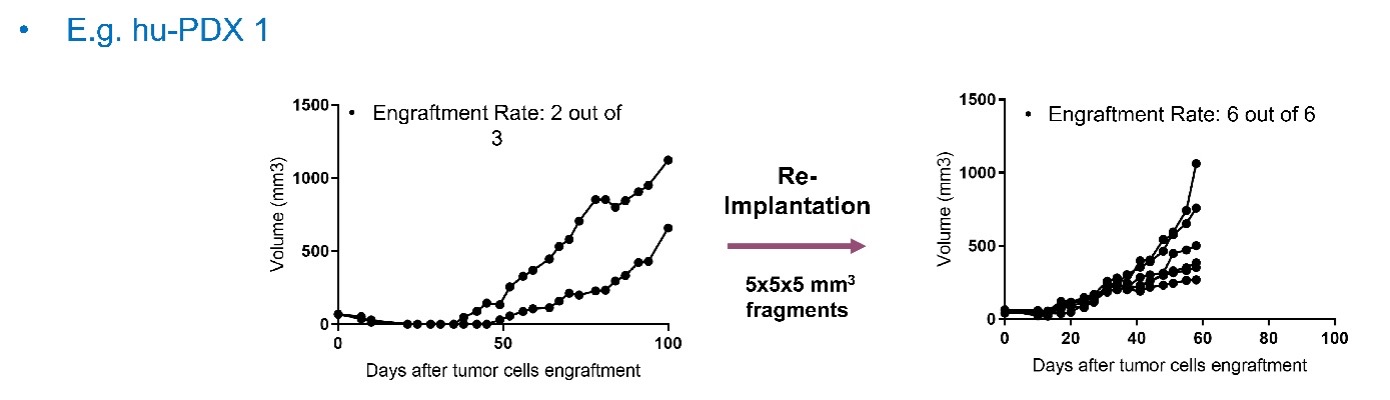
When the average tumor volume of the mice reaches a certain threshold defined in the study protocol, the mice are randomized into multiple treatment groups based on their level of humanization, cord blood donor, and tumor volume. Treatment is then administered at predetermined doses and frequencies for each group.
The subsequent steps of the protocol depend on the specific needs of the study. For example, there may be multiple in-life blood samplings conducted at predetermined time points, including plasma extraction and flow cytometry analyses.
Throughout the study, we offer the possibility to test, follow and evaluate different biological mechanisms and actions of various drugs or drug combinations in CD34+ humanized mice:
- Monitoring of tumor volume & analysis of tumor growth inhibition (score…)
- Monitoring & analysis of drug toxicity (efficacy/profiling) with pharmacokinetic (PK) and pharmacodynamic (PD) in vivo studies
- Flow Cytometry & Immune Profiling (immune phenotype, biomarkers expression, cytokines analysis, therapeutic efficacy, etc.)
- Immunohistological analysis
- Genetic expression profiling
- In Vivo Imaging Newton capacity – Bioluminescence & Fluorescence Imaging
- Tissue and cellular sampling
- Other analysis can be carried out on request, such as downstream characterization of cancer tissue (protein assays, RNA, DNA etc…)
The choice of model for your study can be defined on the advice of our experts and varies according to the needs of the study, depending on the specific objectives of the research, such as understanding tumor biology, evaluating treatment efficacy and the complexity of the type of cancer.
At TransCure bioServices, our commitment lies in supporting immuno-oncology research and advancing preclinical studies for the better treatment of patients. We offer a comprehensive range of services encompassing efficacy studies, pharmacokinetic analyses, safety evaluations, and personalized medicine approaches.
For more information about our services and collaborations, please do not hesitate to contact us.
Sources:
Okada S, Vaeteewoottacharn K, Kariya R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells. 2019 Aug 13;8(8):889. doi: 10.3390/cells8080889. PMID: 31412684; PMCID: PMC6721637.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022 May 10;20(1):206. doi: 10.1186/s12967-022-03405-8. PMID: 35538576; PMCID: PMC9088152
Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020 Jan 7;13(1):4. doi: 10.1186/s13045-019-0829-z. PMID: 31910904; PMCID: PMC6947974.
Stripecke R, Münz C, Schuringa JJ, Bissig KD, Soper B, Meeham T, Yao LC, Di Santo JP, Brehm M, Rodriguez E, Wege AK, Bonnet D, Guionaud S, Howard KE, Kitchen S, Klein F, Saeb-Parsy K, Sam J, Sharma AD, Trumpp A, Trusolino L, Bult C, Shultz L. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol Med. 2020 Jul 7;12(7):e8662. doi: 10.15252/emmm.201708662. Epub 2020 Jun 24. PMID: 32578942; PMCID: PMC7338801.
Landgraf M, McGovern JA, Friedl P, Hutmacher DW. Rational Design of Mouse Models for Cancer Research. Trends Biotechnol. 2018 Mar;36(3):242-251. doi: 10.1016/j.tibtech.2017.12.001. Epub 2018 Jan 5. PMID: 29310843.
Horowitz NB, Mohammad I, Moreno-Nieves UY, Koliesnik I, Tran Q, Sunwoo JB. Humanized Mouse Models for the Advancement of Innate Lymphoid Cell-Based Cancer Immunotherapies. Front Immunol. 2021 Apr 22;12:648580. doi: 10.3389/fimmu.2021.648580. PMID: 33968039; PMCID: PMC8100438.
Tsumura R, Koga Y, Hamada A, Kuwata T, Sasaki H, Doi T, Aikawa K, Ohashi A, Katano I, Ikarashi Y, Ito M, Ochiai A. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci. 2020 Sep;111(9):3386-3394. doi: 10.1111/cas.14564. Epub 2020 Jul 28. PMID: 32639672; PMCID: PMC7469811.
