The utilization of animals for scientific research is governed by specific legislation that has evolved to acknowledge the sensitivity of animals. It is now essential and mandatory to address the pain and suffering arising from experimental conditions (European Parliament & Council Of The European Union, 2010), requiring the ability to detect their signs.
Mice are the most commonly used species in animal experimentation. As prey animals, even when handled with utmost care, laboratory mice perceive humans as potential predators and modify their behavior accordingly. Concealing any sign of weakness from a presumed threat is an instinctual defense mechanism for prey, rendering the detection of pain a challenging task (Turner, 2019). Despite this inherent challenge, various strategies have been developed to identify pain in mice, encompassing clinical signs, application of the Grimace Face Scale, physiological measurements, or behavioral cues.
Examples of clinical signs to watch for in laboratory mice
Certain clinical signs are readily apparent, such as vocalizations, withdrawal from a painful touch, lameness, or excessive scratching. Recognition of these signs serves as compelling evidence of significant pain, prompting the need for immediate intervention through treatment (including analgesics) or, in extreme cases, euthanasia to alleviate the animal’s suffering. To assess the level of pain of an animal, clinical scores prove invaluable, as pain and/or distress can manifest in alterations to its general condition.
A clinical score comprises a catalog of observable indicators in mice, tailored to accommodate diverse research topic and potential inconveniences experienced by the animals. The following non-exhaustive list of signs can be considered (Guide for the Care and Use of Laboratory Animals et al., 2011):
- State of the coat: similar to that of our pet cats, a mouse that neglects grooming experiences
a rapid deterioration in the state of its coat - Paleness: in albino mice, attention should be directed to the color of the ears, legs, and tail
- Movement: abnormally slow movements or difficulty in limb mobility
- Activity: hyperactivity or hypoactivity
- Weight: swift weight loss should be regarded as a concerning sign
- State of stools: the presence of diarrhea may be linked to stress (Julio-Pieper et al., 2012;
Zhang et al., 2021)
At TransCure bioServices (TCS), a clinical score is employed to monitor the overall health status of all animals, comprising 5 signs scored on a scale from 0 to 3. The threshold for concern is defined by a cumulative score of 7.
Each sign is noted as either absent or present (sometimes with degrees such as moderately and strongly present) with an associated score. The sum of scores for these signs provides a general clinical score that can be used as an end point.
Analyzing pain on mice using facial expressions’s indicator
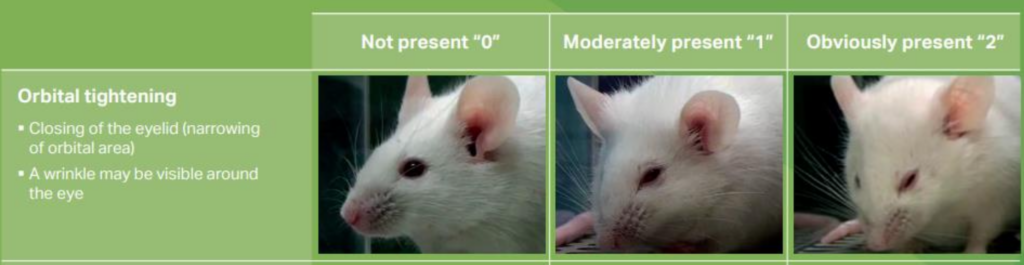
A code for facial expressions of pain was proposed in 2010 for laboratory mice, based on the principle that many animals are capable of expressing emotions through facial expressions (Langford et al., 2010). This code, called “the Mouse Grimace Scale” has become popular and is now widely accepted and used (Whittaker et al., 2021).
Five elements of facial expression related to pain have been selected and must be rated based on their absence (score 0, indicating no pain) up to their moderate (score 1) or intense (score 2) presence. The use of this score requires observation on an alert animal by a person accustomed to contact with mice and over a sufficiently long time. It is an important clinical index of pain but can generate significant inter-observer differences.
At TCS, all staff members in contact with the animals are well-acquainted with the grimace face scale and proficient in detecting these facial expressions in mice.
Physiological measurements: vital signs as indicators
Physiological measures tied to the stress response, such as corticosterone levels (a stress hormone), heart rate (elevated in instances of discomfort), and glucose levels, offer valuable insights (Balcombe et al., 2004). While traditional approaches often involve invasive procedures such as blood tests, emerging techniques, like those presented by Ataallahi et al. (2022), permit the non-invasive measurement of corticosterone in feces, urine, or hair. Additionally, advancements in technology, exemplified by Fares & Boire (2020), have introduced non-invasive vests for monitoring heart rate, presently available for rats and on the horizon for adaptation to mice.
Behavioral cues: anxiety and anhedonia
Excessive scratching or prostration are overt indicators of discomfort or pain behaviors that can be incorporated into clinical scores. These manifestations signify an already progressed state of malaise, while other behavioral signs enable the detection of stress at earlier stages, potentially associated with pain.
Numerous behavioral tests have been devised for laboratory rodents to investigate individual anxiety levels. Usually, a range of devices, more or less anxiety-inducing, is employed. This involves isolating an individual for a variable duration, typically between 5 minutes and 1 hour, while quantifying specific behaviors exhibited by the animal (Durst et al., 2021).
At TCS, we carefully select behavioral anxiety tests from the extensive range available in the literature to minimize their impact on mice. The chosen devices include the open field and the black and white box (See Figure 2), and the test protocol has been streamlined to a minimum duration (5 minutes per mouse) to reduce the isolation time for these social animals.
In addition to anxiety, other behavioral clues are associated with stress and pain. In studies focusing on depression, the sucrose preference test is frequently employed to measure anhedonia (Liu et al., 2018). Typically, this test necessitates isolating mice for a relatively extended period (ranging from 24 hours to a few days). To mitigate any inconvenience to the mice, our internal protocol has been modified to yield results at a cage level rather than on an individual basis.

Advancing pain detectors and management in laboratory mice
Despite the difficulties in detecting pain in mice due to their prey status, several elements have been developed to refine methods in animal experiments. The use of several methods allows for a more reliable assessment; methods using subjective assessment (the clinical score and the Grimace Face Scale) require experienced observers accustomed to contact with mice.
The ability to detect pain in laboratory mice is not sufficient for its management, which is hampered by other elements(Foley et al., 2019):
- The fear of impacting the scientific results of the study with the use of analgesics. In fact, most have an anti-inflammatory effect and can interact with the physiology of the animal, or the treatments administered.
- The metabolism of mice is particularly rapid, and the animals recover quickly from the procedures inflicted on them. Thus, there is a belief that mice need little or no analgesia, or over a short period of time.
However, untreated pain is a significant bias in research and can alter scientific results through the interaction between the stress generated by this pain and the physiology of the animal, in particular its immune system (Taylor 2019).
To refine our methods in the context of using animals for scientific purposes, we must continue to improve pain detection methods but also reduce the obstacles to its management. Using analgesic treatments preventively (without waiting to observe a sign of pain, for example before surgery) or even when only suspecting pain (without observing a sign of pain, we can consider that a lesion such as an ulcerated tumor is at least uncomfortable, certainly painful) are necessary advances that must be generalized.
For instance, at TCS, a mouse displaying a tumor at the pre-ulcerative phase (such as redness without skin lesions) is subjected to analgesic treatment.
Our knowledge and consideration of the pain experienced by the animals we use for scientific purposes improves over time. We must continue this evolution towards refinement of all of our pain management strategies in laboratory mice.
Bibliography
Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and LifeStudies, & National Research Council. (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. In National Research Council 2011 (Ed.) National Academies Press.
Durst, M. S., Arras, M., Palme, R., Talbot, S. R., & Jirkof, P. (2021). Lidocaine and bupivacaine as part of multimodal pain management in a C57BL/6J laparotomy mouse model Scientific Reports, 11(1).
EUROPEAN PARLIAMENT, & COUNCIL OF THE EUROPEAN UNION. (2010). DIRECTIVE 2010/63/EU.
Foley, P. L., Kendall, L. V., & Turner, P. V. (2019). Clinical Management of Pain in Rodents In Comparative Medicine (Vol. 69, Issue 6, pp. 468–489). American Association for Laboratory Animal Science.
Julio-Pieper, M., O’Mahony, C. M., Clarke, G., Bravo, J. A., Dinan, T. G., & Cryan, J. F. (2012). Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent Stress, 15(2), 218–226.
Liu, M. Y., Yin, C. Y., Zhu, L. J., Zhu, X. H., Xu, C., Luo, C. X., Chen, H., Zhu, D. Y., & Zhou, Q. G. (2018). Sucrose preference test for measurement of stress-induced anhedonia in mice Nature Protocols, 13(7), 1686–1698.
NC3Rs. (2015, September 1). NC3Rs ressources, The Mouse Grimace Scale.
Zhang, C. Y., Peng, X. X., Shao, H. Q., Li, X. Y., Wu, Y., & Tan, Z. J. (2021). Gut Microbiota Comparison Between Intestinal Contents and Mucosa in Mice With Repeated Stress-Related Diarrhea Provides Novel Insight Frontiers in Microbiology, 12.