Liver cancer, specifically hepatocellular carcinoma (HCC), stands as a major global health concern, ranking as the sixth most prevalent cancer and the fourth leading cause of cancer-related mortality. The disease’s etiology is complex, with varied risk factors including hepatitis infections, lifestyle-related conditions such as diabetes and obesity, and genetic predispositions. This blog post delves into the multifaceted origins of liver cancer and the mechanisms driving its development. It also explores the latest advancements in treatment options, underscoring the importance of accurate and predictive preclinical mouse models to test innovative treatments.
Liver cancer: etiology and physiopathology
Liver cancer is a global health challenge as it is the 6th most widespread type of cancer in the world and the 4th cause of cancer-related deaths1. Among liver diseases, hepatocellular carcinoma (HCC) represents 90% of cases2. First originated from East Asia and Africa, this disease is growing in Europe and North America in relation to increasing overweight and alcohol consumption1,3.
Liver cancer occurs in a chronic inflammatory state, which can be originated from several conditions. Hepatitis B or C virus infection (HBV, HCV), cirrhosis, diabetes, non-alcoholic steatohepatitis (NASH) or non-alcoholic fatty liver disease (NAFLD) and exposure to aflatoxin B1 are all factors of risk of liver cancer. Interestingly, the incidence of HCC differs according to the geographical area and level of development. In countries with a low vaccination coverage, HBV is a leading cause of HCC, while in Western countries, HCC is more related to HCV infection or NASH1,3,4.
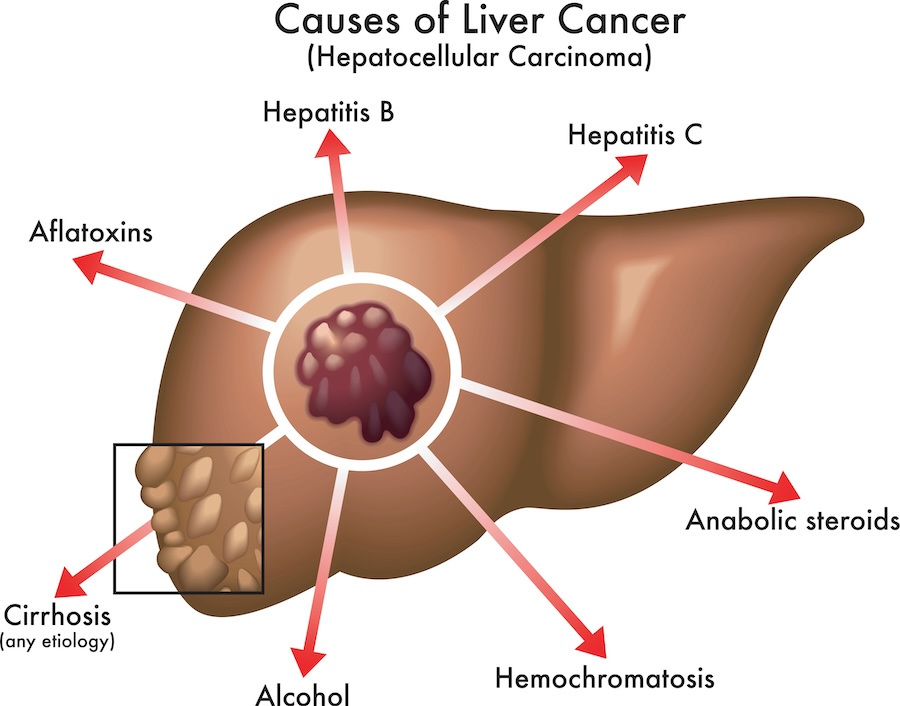
Several mutations have been identified in patients with HCC, the most common being on the TERT gene for around 60% of patients, which can be caused by HCV infection. Also, a mutation on the CTNNB1 gene, supposed to enhance tumor proliferation, was reported in 40% of liver tumors. In addition, HBV infection and chronic exposure to aflatoxins (mycotoxins produced by Aspergillus fungi, mainly found in cereals in Southeast Asia) are known to induce mutations on the TP53 gene, which are found in many cancers, including HCC. Indeed, these mutations are responsible for a downregulation of the immune response, which is related to poor clinical outcomes. HBV and HCV infection are also thought to contribute to mutations on the CDKN2A gene which is involved in tumor suppression, such mutations being therefore favorable to HCC development1,3.
Apart from genetic mutations, metabolic dysfunction observed in patients with diabetes or NASH/NAFLD can also be responsible for the apparition of liver cancer, through the strong inflammatory state causing tissue lesions. In patients with obesity, microbiota-associated molecular patterns (MAMPs) moving from the gut to the liver, as well as chemokine CC motif ligands (CLC) produced by adipose tissue, activate hepatic stellate cells which causes fibrosis and favors liver cancer. Patients with insulin resistance are another example of people being more susceptible to liver cancer, due to overstimulation through NF-kB and STATs signaling pathways following activation of adipose tissue and ROS production3.
Recently, the role of tumor microenvironment (TME) has gained much interest in the understanding of cancer progression. Liver tumors grow in an immunosuppressive TME and are surrounded by immune cells, especially lymphocytes which participate to tumor invasion and metastasis when some pathways are activated5. Treg cells which are found in the TME are known to downregulate immune activity and inhibit cytotoxic T cells. In addition, activated Kupfer cells can also play an immunosuppressor role by releasing IL10 and TGFβ anti-inflammatory cytokines, by expressing PDL1 to suppress T cell activity, and by promoting angiogenesis, fibrogenesis and carcinogenesis through the production of osteoponin. Moreover, macrophages attracted by HCC tumor cells in the TME differentiate into an M2-profile which is associated with angiogenesis and metastasis1,3.
Treatment of liver cancer
The choice of treatment for liver cancer is based on the Barcelona Clinic Liver Cancer staging system (BCLC), which is recommended by both the European and the American Association for the Study of Liver Diseases3. The BCLC takes into consideration the number and size of tumors in the liver, the general health of the patient, as well as the liver function6. Indeed, the treatment must be adapted to the stage of cancer development. For this reason, an early diagnostic is of great importance for the fate of patients. People with HBV or cirrhosis are under surveillance, however there are still 50% patients diagnosed too late1. The difficulty remains in the lack of reliable biomarkers specific for liver cancer. High levels of alpha-fetoprotein (AFP) can be detected in the serum of patients with HCC, which is already a synonym of poor prognosis. Other recently discovered biomarkers could be used in the future, such as Golgi 73 protein (GP73) and Glypican-3 (GPC3)4.
Although the management of HCC has much improved since 2010, therefore increasing the patient’s survival, there is still a great need for innovative treatments, especially to prevent cancer relapse1. For patients with early-stages HCC, surgical resection, radiofrequency ablation or transplantation are recommended curative options. Surgical resection is more specific to patients without cirrhosis and because this technique is not very effective at preventing tumor recurrence, it is not feasible in case of metastases. Radiofrequency ablation consists in the destruction of tumors smaller than 5cm, which also induces ischemia4. This treatment is usually better tolerated by the patients than surgical resection. For early-stage HCC with cirrhosis, patients receive a transplantation, which prevents recurrence and improves survival.
For the treatment of intermediate stage HCC, arterial chemoembolization is indicated. Embolization of the hepatic arteria is performed using a chemotherapeutic agent, usually doxorubicin or cisplatin, which interrupts the tumor blood supply while avoiding a systemic exposure to chemotherapeutics4.
Then, for more advanced stages HCC, with extrahepatic spread but preserved liver function, systemic treatments are recommended. Sorafenib, an oral multiple kinase inhibitor was approved in 2007 for the treatment of liver cancer and it remained the only treatment for advanced HCC until the approval of Lenvatinib, another multiple kinase inhibitor, in 20181,3,4. These molecules are effective through their anti-angiogenic properties, by inhibiting the vascular endothelium growth factor VEGF-2. In second-line, Regorafenib and Cabozantinib, two other inhibitors of multiple kinase, as well as Ramucirumab, a monoclonal antibody targeting the receptor of vascular endothelium growth factor VEGFR2, are used to treat patients who did not respond to Sorafenib or Lenvatinib1,3.
To improve treatment efficacy, combination regimens are emerging, such as immune checkpoint inhibitors associated with anti-angiogenic agents, tyrosine kinase inhibitors, or other monoclonal antibodies1. Such combination therapies are applied to 50 to 60% of patients with liver cancer. Indeed, it was recently demonstrated that the combination of Atezolizumab (anti-PDL1) and Bevacizumab (anti-VEGF) improved the patient’s survival compared to Sorafenib, while only 15-20% patients respond to immune checkpoint inhibitors when used alone.
More effective therapies are still awaited and for this purpose, new targets are considered. However, most known mutations on liver cancer cells have a low prevalence, which prevents their use as therapeutical targets. Nonetheless, other molecules or actors can be of interest. For example, Treg cells immunosuppressive activity is mediated by TGFβ and IL10 which could be targeted to promote immune checkpoint inhibitors efficacy1. The role of tumor associated neutrophiles (TAN), through the expression of CCL4 and CXCR2 molecules is also under investigation5. Moreover, new molecular biomarkers have recently been identified in HCC and could be potential therapeutic candidates, such as GPC3 (Glypican-3), OPN (Osteopontin), GP73 (Golgi Protein 73), VEGF, EGF (Epidermal Growth Factor), PDGF gene (Platelet Derived Growth Factors), IGF (Insulin-like Growth Factors), mTOR (mammalian Target of Rapamycin), and microRNAs4.
Animal models for the study of liver cancer
The establishment of animal models for the study of liver diseases and the evaluation of novel therapies is a key step to bring promising therapies to the clinic. The main platforms are genetically engineered mice, chemically-induced mice and implantation models. Genetically engineered mouse (GEM) models are based on the silencing of tumor suppressor genes, or the overexpression of oncogenes, growth factors or viral genes which are known to induce HCC7. Such models are of interest to study the origin of HCC, especially the involvement of specific genes or pathways in pathophysiological changes. Indeed, HCC develops spontaneously from the induced genetic mutations. The main techniques used to establish GEM models are Cre-Lox recombination, CRISPR-Cas9, Sleeping Beauty transposon system or stem cell transduction8,9. However, the GEM models lack the induction of fibrosis or cirrhosis, which is often present in patients with HCC. Also, tumors are originated from little genetic modifications, while HCC usually develops in a context of various gene alterations7. Moreover, these models require a lot of time to generate diseased mice.
There are two kind of molecules which can cause HCC. Genotoxic carcinogens, such as diethylnitrosamine (DEN) or aflatoxins, directly induce DNA damage and a chronic exposure to them would result in chronic inflammation followed by fibrosis7,9. The mechanisms behind the progression of liver cancer are very close to those in patients, which makes this model very attractive for the study of the pathogenetic alterations involved. However, DEN-induced HCC is highly dependent on the strain, age and sex of mice used, and a prolonged exposure to the chemical is required to generate tumors. Aflatoxin is known to be a high-risk factor for liver cancer development in humans, but it is rarely used in mice as there is a strong variability in the susceptibility to develop HCC depending on mouse strains, and tumors take a several months to develop8. Non-genotoxic carcinogens can also be used as inducers of HCC, namely carbon tetrachloride (CCl4), thioacetamide (TAA) as well as phenobarbital. These molecules cause hepatic damage, resulting in fibrosis and then HCC. Combination of DEN and CCl4 leads to more reproducible and shorter-lasting models7.
The most frequently used method to establish HCC is based on the engraftment of cancer cells or tissues in mice. These models are often well established, reproducible and rather easy to use. Tumor cells or tissues are mainly engrafted subcutaneously, which allows the easy tumor growth monitoring but does not properly recapitulate the tumor microenvironment. Therefore, orthotopic models which consist in the engraftment of tumor cells in the liver through intrahepatic, intrasplenic or intraportal injection, are more accurate in reflecting the interactions between the tumor and the surrounding tissues9. These models are of great interest as the liver is both a metabolic organ and a key immune tissue, which makes its tumor microenvironment very complex8. In this case, tumor growth can be monitored by bioluminescence imaging using luciferase-expressing cancer cell lines. Syngeneic models consist in the engraftment of murine tumor cells in immunocompetent mice. These models allow the evaluation of new therapeutics and study the involved immune responses, however the major limitation is the genetic differences with humans. On the contrary, xenograft models are immunocompromised mice inoculated with human tumor cells or tissues, which is required to prevent tumor rejection. Although it provides a useful tool to assess new treatment strategies, the major drawback of these models is that a functional immune system is missing, thus it is not reflective of the process of tumor immune surveillance7.
Humanized mouse models are of great potential, as they are reconstituted with human immune cells, and would therefore allow the study of interactions between both human immune cells and tumor cells. Humanized mice are generated by the engraftment of human peripheral blood mononuclear cells (PBMC), human CD34+ hematopoietic stem cells (HSC) or fetal liver and thymus fragments to highly immunodeficient mice. Such mice models were used to evaluate the efficacy of promising immunotherapy drugs, for example STAT3 inhibitor C1889, VEGF inhibitor bevacizumab, PD-1 antibody pembrolizumab, or CTLA-4 inhibitor ipilimumab8. The “cherry on the cake” would be to establish an orthotopic model of liver cancer in mice with both their immune system and their liver humanized. This would be the best platform for the close study of the interactions between tumor, liver and immune cells, all of human origin. A mouse model with humanized liver and immune system has already been described10 but its use in HCC research is yet to come.
Summary:
This comprehensive overview highlights the intricate nature of liver cancer, the different treatment options, and the evolving landscape of preclinical mouse models, all of which contribute to a more hopeful prognosis for patients battling this disease.
Interested to learn more? Do not hesitate to contact us!
Bibliography
-
Llovet, J. M.; Kelley, R. K.; Villanueva, A.; Singal, A. G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R. S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2021, 7 (1), 1–28.
-
Llovet, J. M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2016, 2, 16018.
-
Méndez-Sánchez, N.; Valencia-Rodríguez, A.; Coronel-Castillo, C. E.; Qi, X. Narrative Review of Hepatocellular Carcinoma: From Molecular Bases to Therapeutic Approach. Dig. Med. Res. 2021, 4 (0).
-
Tunissiolli, N. M.; Castanhole-Nunes, M. M. U.; Biselli-Chicote, P. M.; Pavarino, É. C.; da Silva, R. F.; da Silva, R. de C. M. A.; Goloni-Bertollo, E. M. Hepatocellular Carcinoma: A Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac. J. Cancer Prev. APJCP 2017, 18 (4), 863–872.
-
Liao, W.; Calvisi, D. F.; Chen, X. Year in Review: Liver Cancer Research in 2022: Tumor Microenvironment Takes the Central Stage. Hepatol. Commun. 2023, 7 (3), e0074.
-
Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R. K.; Galle, P. R.; Mazzaferro, V.; Salem, R.; Sangro, B.; Singal, A. G.; Vogel, A.; Fuster, J.; Ayuso, C.; Bruix, J. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76 (3), 681–693.
-
Macek Jilkova, Z.; Kurma, K.; Decaens, T. Animal Models of Hepatocellular Carcinoma: The Role of Immune System and Tumor Microenvironment. Cancers 2019, 11 (10), 1487.
-
Zheng, H.; Xue, H.; Yun, W.-J. An Overview of Mouse Models of Hepatocellular Carcinoma. Infect. Agent. Cancer 2023, 18 (1), 49.
-
Brown, Z. J.; Heinrich, B.; Greten, T. F. Mouse Models of Hepatocellular Carcinoma: An Overview and Highlights for Immunotherapy Research. Nat. Rev. Gastroenterol. Hepatol. 2018, 15 (9), 536–554. https://doi.org/10.1038/s41575-018-0033-6.
-
Bility, M. T.; Zhang, L.; Washburn, M. L.; Curtis, T. A.; Kovalev, G. I.; Su, L. Generation of a Humanized Mouse Model with Both Human Immune System and Liver Cells to Model Hepatitis C Virus Infection and Liver Immunopathogenesis. Nat. Protoc. 2012, 7 (9), 1608–1617.