Discover the innovation of CAR-T therapies, designed to specifically target cancer cells, showing remarkable efficacy against leukemias and lymphomas. This first blog of a two part series explores the different generations of CAR-T and the autologous, allogeneic, and in vivo approaches of this groundbreaking therapy.
What is CAR-T cell therapy?
CAR-T (chimeric antigen receptor T-cell) therapies are a type of immunotherapy that uses genetically engineered T cells to treat cancer. T cells are modified to express a chimeric antigen receptor (CAR) on their surface which allows them to recognize and bind to specific proteins, or antigens, present on cancer cells. Once bound, the CAR-T is activated and can destroy the cancer cells. CAR-T cell therapies have shown remarkable effectiveness in treating certain types of blood cancers, such as B cell leukemia and lymphoma. They are also being investigated for their potential in treating solid tumors and other diseases.
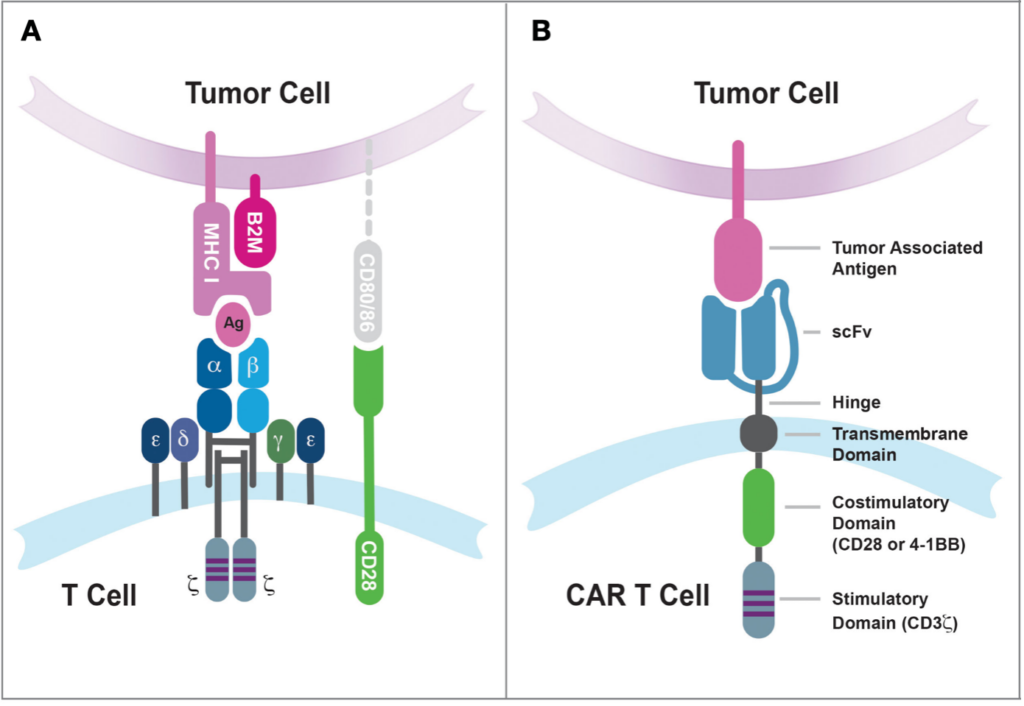
The basic molecular structure of a CAR consists of four main components expressed exogenously into T cells :
- Extracellular target antigen-binding domain : This domain is responsible for recognizing and binding to the target antigen on cancer cells. It is typically derived from a single-chain variable fragment (scFv) of a monoclonal antibody.
- Hinge region : It connects the extracellular domain to the transmembrane domain. It provides flexibility and allows for optimal binding of the CAR to the target antigen
- Transmembrane domain : It anchors the CAR to the T cell membrane, helps stabilize the receptor, and is involved in CAR-T cell function
- Intracellular signaling domain(s) : This domain is responsible for transmitting signals to the T cell upon binding of the CAR to the target antigen. It typically contains signaling domains derived from CD3ζ (T-cell receptor T3 zeta chain or CD247, which is involved in T cell activation), and later generations also include co-stimulatory domains such as CD28 or 4-1BB to enhance CAR-T cell function
Major milestones in CAR-T development and clinical approval
The first-generation CAR-Ts contains a single signaling domain, typically CD3ζ and had limited persistence and efficacy in clinical trials. These limitations prompted the development of a second generation of CARs containing a costimulatory-domains, such as CD28 or 4-1BB, in addition to the CD3ζ signaling domain. This drastically improved T cell activation, proliferation, and cytotoxicity compared to first-generation CAR-T cells and, since 2017, has led to 12 FDA approvals for 6 distinct CAR-T products, all of which being second-generation. Approved indications include relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, relapsed or refractory mantle cell lymphoma (MCL), B-cell precursor acute lymphoblastic leukemia (ALL), and relapsed or refractory multiple myeloma (MM).
Out of the 6 clinically-approved CAR-T therapies, 4 target CD19 :
- Tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corporation, approved in 2017)
- Axicabtagene ciloleucel (Yescarta, Kite Pharma/Gilead Sciences, approved in 2017)
- Brexucabtagene autoleucel (Tecartus, Kite Pharma/Gilead Sciences, approved in 2020)
- Lisocabtagene maraleucel (Breyanzi, Bristol Myers Squibb, approved in 2021)
And 2 target B-cell maturation antigen (BCMA) :
- Ciltacabtagene autoleucel (Carvykti, Janssen Biotech, Inc, approved in 2020)
- Idecabtagene vicleucel (Abecma, Bristol Myers Squibb, approved in 2021)
Given the clinical breakthrough of the second generation of CAR-Ts, a third generation was developed which includes multiple (and hypothetically complementary) costimulatory domains (most frequently combining CD28 which drives quicker expansion and more rapid elimination of tumor cells with 4-1BB which is associated with longer persistence) in the same CAR while, in some instances, targeting novel antigens such as CD20 (a.k.a. MS4A1), CD22 and CD123 in hematological malignancies and TAAs, as well as HER2 and GD2 in solid tumors. Although third generation CAR-Ts had improved post-infusion persistence and expansion compared to the second generation, preclinical and clinical trials are still undergoing to identify sufficient improvements over existing CAR therapies.
In addition, new features in what have been called fourth-, fifth-, or more simply next-generation CARs are being investigated and encompass a diverse group of constructs that aim at circumventing the inhibitory stimulation in the tumor microenvironment and/or harnessing it to achieve higher tumor infiltration and cancer cell killing, including:
- Armored CARs, which contain immune checkpoint modulation elements (e.g. PD-1 or PDL-1 derivatives) thus resisting immunosuppressive tumor signals
- TRUCKs (T cells Redirected for Universal Cytokine-mediated Killing), which are engineered to express certain cytokines (e.g. IL-7, IL-12, CC19)
- Self-driving CARs, which express a chemokine receptor connecting to tumor-secreted chemokines
- Switchable CARs, which can be activated or deactivated by administration of external agents
Autologous, allogeneic, and in vivo CAR-T approaches
CAR-T therapy can be autologous or allogeneic, the former being the more clinically tested and approved approach. Autologous CAR-T therapy involves collecting T cells from the patient’s own body, genetically modifying them to express CARs, and then reinfusing them back into the same patient. Autologous CAR-Ts have been approved for the treatment of various B-cell malignancies, including B-cell lymphomas and B-cell acute lymphoblastic leukemia. However, autologous CARs have several limitations including a great cost (up to $500,000 per patient), considerable manufacturing time (20-35 days, during which the patient may require bridging therapy or have life-threatening disease progression especially if they’ve received prior treatments), and high exhaustion status of the patient’s own T cells (compared to less exhausted, more active T cells from healthy donors). For these reasons, significant research has gone into developing alternative approaches such as off-the-shelf, more affordable allogeneic CAR-T cells and in vivo generation of CAR-T cells.
Allogeneic CAR-T therapy uses T cells from a healthy donor that are modified to express CARs and then infused into the patient. This form of adoptive CAR cell therapy is still being investigated in clinical trials and has not yet been approved for clinical use. While offering the advantage of being a readily available treatment, allogeneic CAR-Ts also presents challenges such as graft-versus-host disease and the risk of rejection by the host immune system leading to poor CAR-T survival and efficacy. Strategies to overcome the limitations of allo-CARs (e.g. via CRISPR/Cas- or TALEN-mediated knockdown of HLA class I/II and T-cell receptors) and improve the safety and efficacy are currently being exploited.
Finally, the generation of CAR-T cells in vivo has the potential of bypassing ex vivo T-cell engineering and therefore reducing time and cost. To that end, several approaches are currently being developed including LNP-mediated mRNA delivery, which leads to transient CAR-T presence in vivo, and lentiviral transduction for persistent CAR-T production in the patient.
Summary
CAR-T therapies have revolutionized the game in cancer treatment. From their first-generation models that pioneered the path, through the robust and efficient second generation that birthed six clinically approved therapies, to the advent of the dynamic third generation and beyond, each phase of development represents an exciting leap forward.
In our next blog, we’ll delve deeper into the CAR-T cell therapy challenges and how you can navigate through it by choosing the right preclinical mouse models.
Interested to learn more? Do not hesitate to contact us!
References :
Alnefaie et al. Chimeric Antigen Receptor T-Cells: An Overview of Concepts, Applications, Limitations, and Proposed Solutions. Frontiers in Bioengineering and Biotechnology (2022)
Cappell and Kochenderfer. Long-Term Outcomes Following CAR T Cell Therapy: What We Know so Far. Nature Reviews Clinical Oncology (2023)
Kagoya et al. Genetic Ablation of HLA Class I, Class II, and the T-Cell Receptor Enables Allogeneic T Cells to Be Used for Adoptive T-Cell Therapy. Cancer Immunology Research (2020)
Mitra et al. From Bench to Bedside: The History and Progress of CAR T Cell Therapy. Frontiers in Immunology (2023)
Scott et al. Trends in the Approval of Cancer Therapies by the FDA in the Twenty-First Century. Nature Reviews Drug Discovery (2023)
Sterner and Sterner. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer Journal (2021)
Tomasik et al. Next Generations of CAR-T Cells – New Therapeutic Opportunities in Hematology? Frontiers in Immunology (2022)
Wang et al. Systematic Review on CAR-T Cell Clinical Trials Up to 2022: Academic Center Input. Cancers (2023)
Wells et al. A Review of CAR T-Cell Therapies Approved for the Treatment of Relapsed and Refractory B-Cell Lymphomas. Journal of Hematology Oncology Pharmacy (2022)