Immuno-Oncology Models
Conventional tumor models with immunodeficient animals only monitor the effect of a drug on the tumor itself. The approval in 2011 of the first immune-checkpoint inhibitor (Nobel Prize Winner in 2018) has validated the immuno-oncology therapeutic approach against many forms of cancer.
TransCure bioServices has developed one of the most predictive preclinical immuno-oncology mouse models. Engraftment of tumor cells lines (CDX) or patient-derived xenografts (PDX) in our CD34+ humanized mouse models fully recapitulate the cancer disease.
Thanks to our strong expertise in CD34+ humanized mouse models, TransCure BioServices can provide custom study designs in order to: analyze PK/PD response of your drug candidates, assess the therapeutic efficacies, and/or decipher their mechanisms of action.
Make sure to check out our complimentary webinar “How Humanized Mouse Models Enforce Preclinical Studies in Immuno-Oncology“, a 2 minute introduction is provided here.
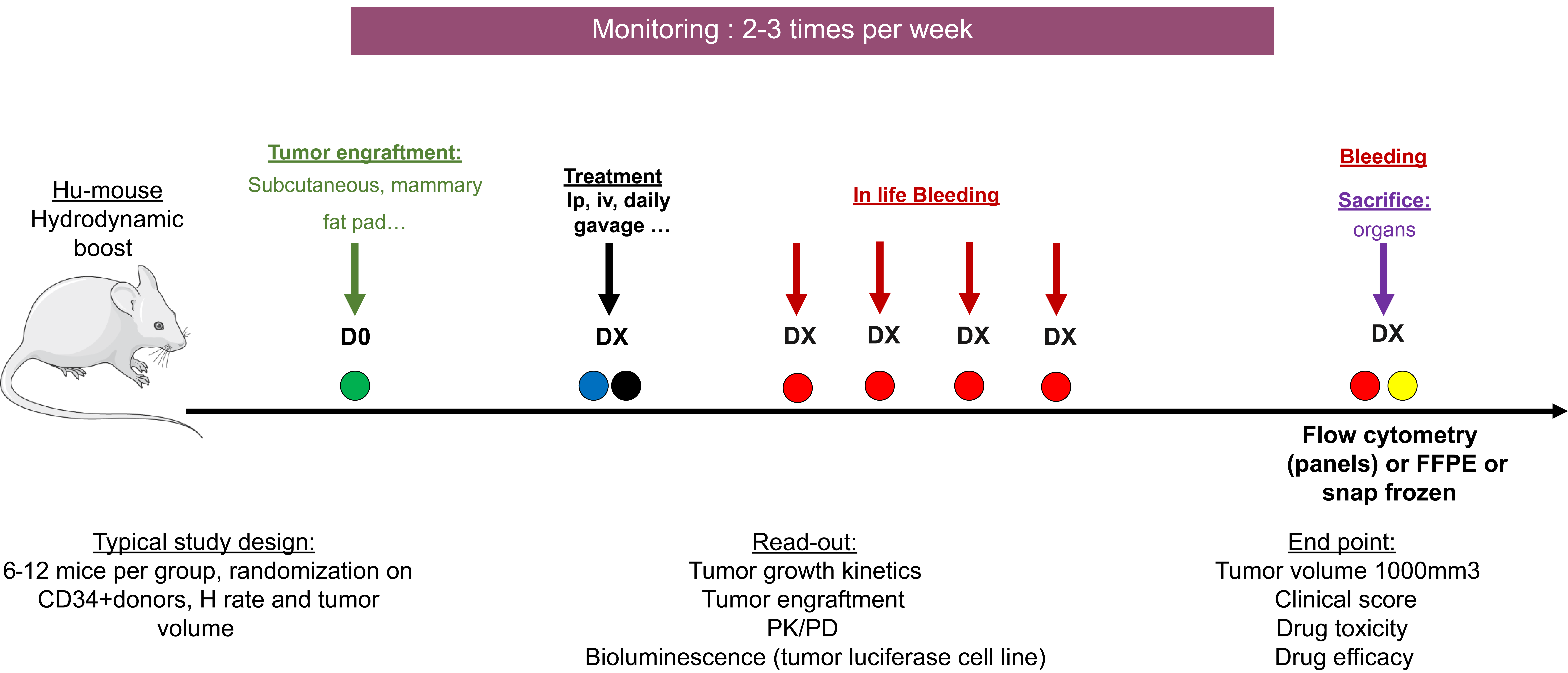
Preclinical IO study
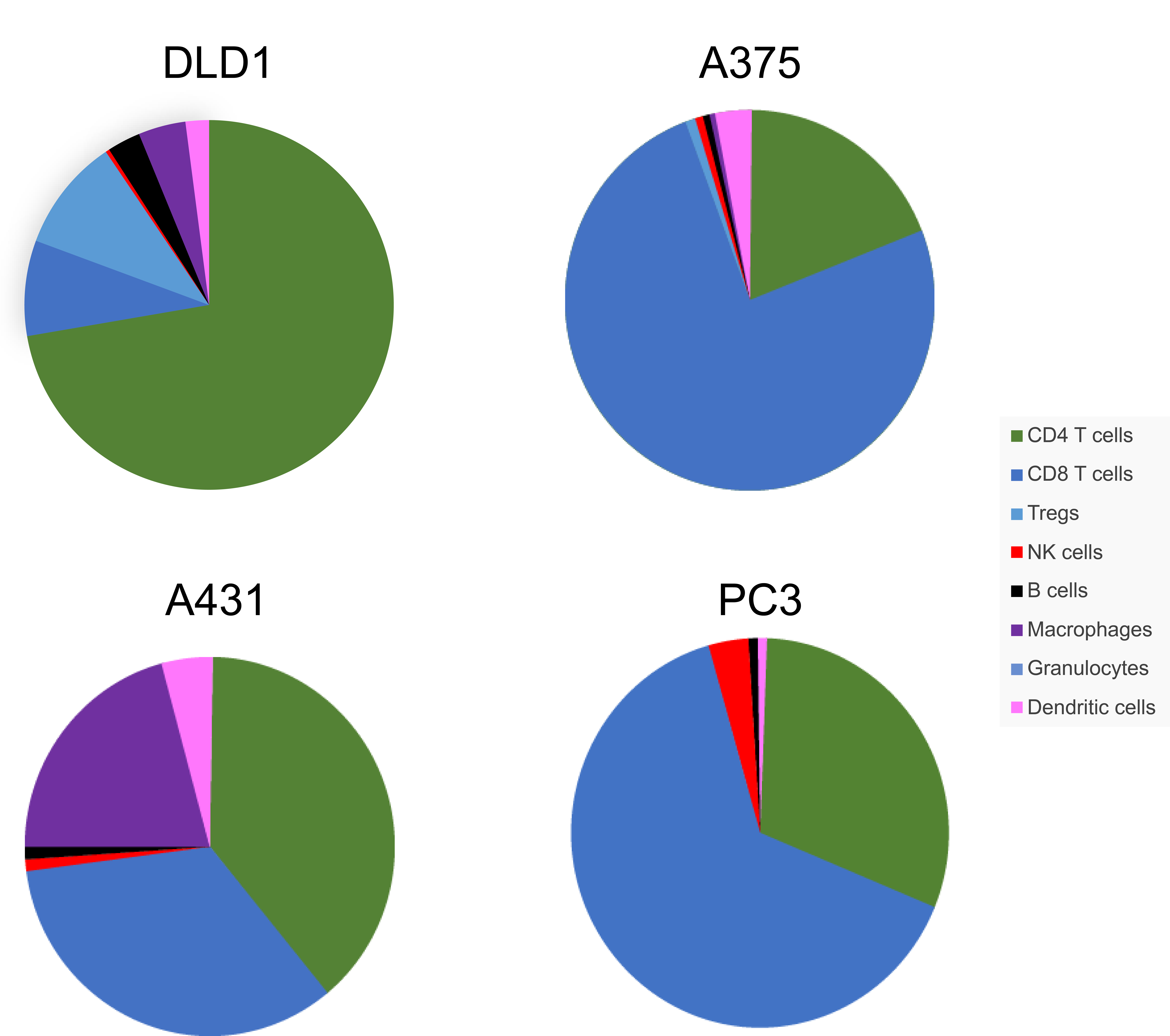
We have a list of fully characterized tumor cell lines and can provide access to their tumor micro-environment (TME) infiltration data, so you can choose the best tumor model for your specific needs.
Tumor Immuno-phenotype
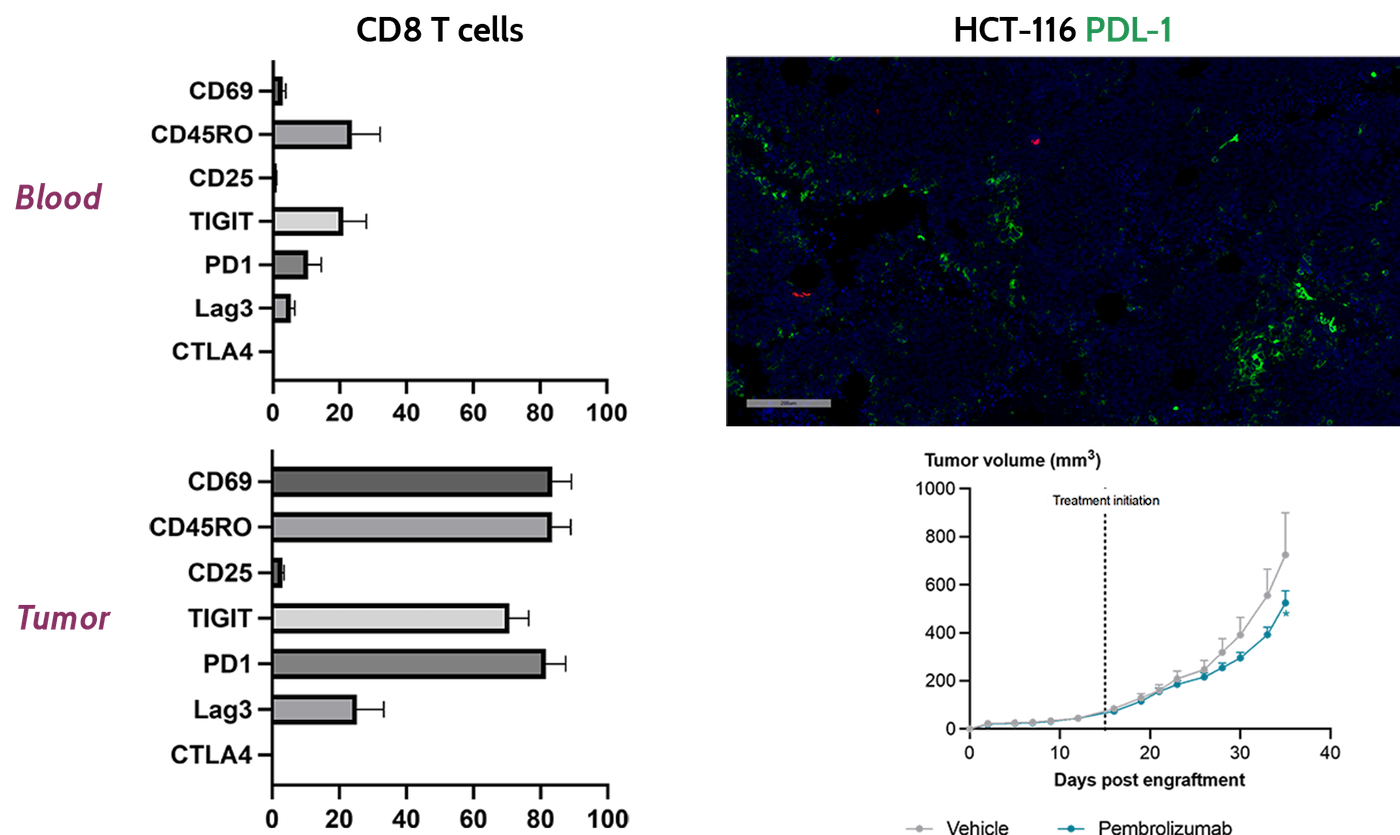
Drug testing in our humanized mouse models will allow you test mechanisms of actions such as: Immune-checkpoint inhibitors (ICP), ADCC, Phagocytosis, T-cell engagers, NK-bispecifics, cytokines, cell therapies, and CAR-based therapies.
Immune-checkpoint inhibitor and T cells exhaustion
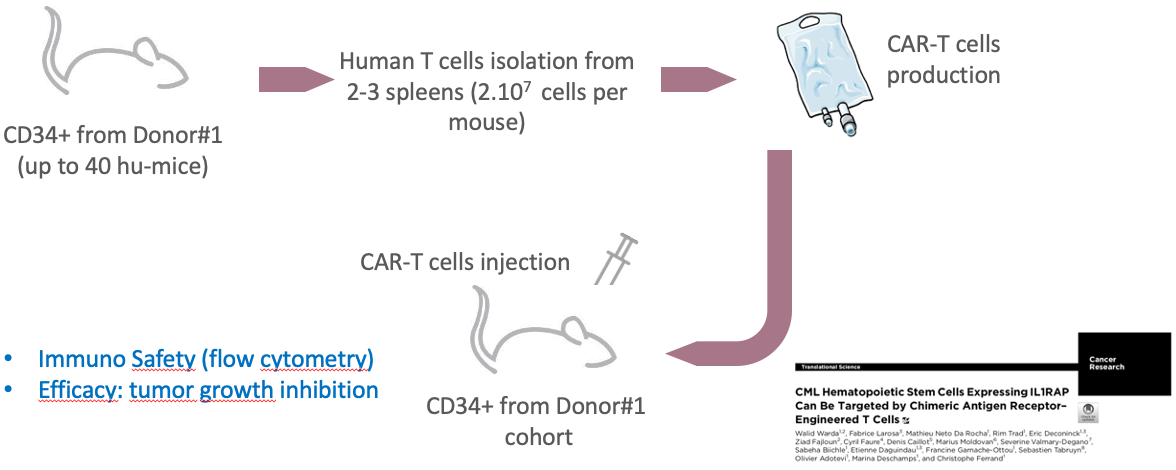
CAR-T cells and hu-mouse models
Read-outs:
- Tumor growth curve
- Flow cytometry
- IHC platform
- In vitro assays
- In vivo imaging
Other mouse models are also available:
- Engraftment of PDX in CD34+ humanized mice
- Engraftment PDX/CDX in immunodeficient mice
- Syngeneic mouse models
- Human PBMC engrafted mouse models