The immune system plays a powerful role in many pathologies including cancer, inflammatory and autoimmune diseases, but it is not fully understood. To develop better treatments for these conditions, it is essential to have accurate predictive pre-clinical models that truly reflect the immune response.
Most studies opt to use mouse models, as their genome is so similar to that of humans. But many pre-clinical models, including mice, lack relevance, leading to key interactions between the human immune system and cells being missed until a later stage. Without the full picture, treatment efficacy is unclear, and significant costs and timeline delays are commonplace as a result. And with almost 90% of novel drugs that pass preclinical tests failing in human clinical trials, new approaches to improve the odds are desperately sought.
Human immune system (HIS) mouse models offer a favorable solution, delivering a more accurate prediction of clinical responses to factors that can trigger an immune response. These models, therefore, have the potential to greatly improve our understanding of the immune system and ensure the more efficient progression of only the most promising treatment candidates — with those likely to fail being eliminated. In this blog post, we discuss the benefits of HIS mice models and explore how they are generated and characterized.
What mouse models are available, and which is best?
There are several mouse models available for immune-targeted studies, each with different strengths and weaknesses. One such model is “humanized mice,” which express anywhere from 1-4 genes of human origin in a mouse system. While these mice do recapitulate the presence of the target, they do not provide insight into how the HIS reacts. Some may consider these as humanized mice, but for our purposes we define HIS mice as ones in which a full human immune system model is recapitulated.
An alternative model involves the engraftment of peripheral blood mononuclear cells (PBMC) into mice, endowing them with a large number of human immune cells. Unfortunately, though, only T cells remain after a few days, and monocyte dendritic cells are lost within a week, leading to the development of graft versus host disease (GvHD). The GvHD reaction not only reduces animal welfare, it can also cause significant variabilities in the obtained research data.
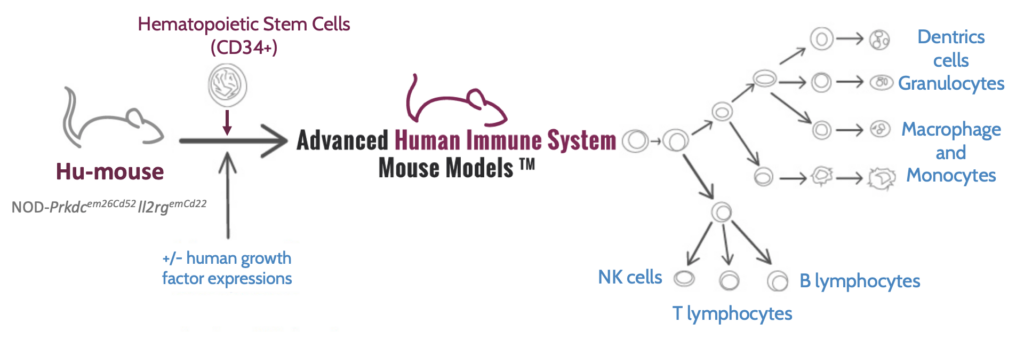
One recently developed model that has gained attention is the CD34+ HIS mouse model, which is the only system where all human immune targets are reconstituted into a single animal. Using the model, researchers can efficiently study the interaction between all different constituents of immune cells to gain significantly deeper insights.
The CD34+ HIS mouse model has several advantages, including stable humanization for the animal’s lifetime (up to a year) and the absence of a GvHD reaction. These characteristics make it a valuable tool for immune system studies. But how are CD34+ HIS mice generated?
Not all mice are made equal
The method used to generate HIS mouse models can significantly affect the quality of the model. For example, some HIS mice are generated through irradiation, which makes room in the bone marrow to allow engraftment of hematopoietic cells. However, the process can induce anemia, leading to lower animal welfare and a shorter lifespan — forcing studies to terminate prematurely.
A preferable approach is chemoablation, in which highly immunodeficient mice are transplanted with CD34+ hematopoietic stem cells from umbilical cord blood. After 14 weeks, a fully functional human immune system is established in the mouse (Figure 1). Chemoablation has several advantages, including no anemia induced, improved welfare, and a more stable immune system.
– – –
Partners that offer mouse models with improved welfare will be Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited.
– – –
The standard of the hematopoietic cells used is also critical to the overall quality of the model. Cells from cord blood have better engraftment properties than those from peripheral blood or bone marrow cells. Cell purity, another significant factor in model quality, must be very high as any T cell contamination can result in GvHD.
In some cases, mice can also undergo human cytokine boosting, in which human cytokines are overexpressed to improve engraftment and reconstitution of human immune cells. However, this can lead to overactivated T cells and subsequent GvHD. An alternative is hydrodynamic gene delivery — where plasmid DNA is delivered in 2 mL of saline — which boosts the number of natural killer (NK), myeloid, and dendritic cells (DCs) and leads to stable differentiation for up to three months (Figure 2).
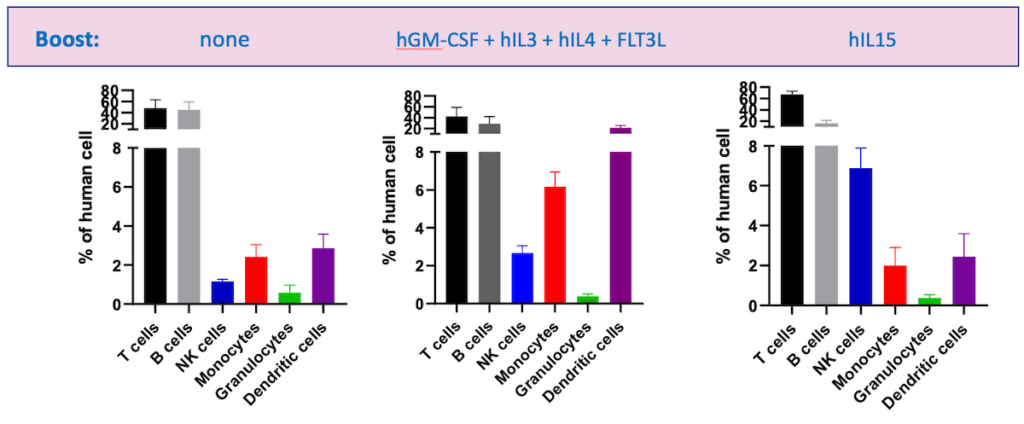
Ensure accuracy in your study
When working with humanized mice, it is crucial that you can rely on your model. The humanization rate, or the ratio between human immune cells and mouse immune cells, is the most significant quality indicator in HIS mouse models — a high-quality mouse model will have an average humanization rate of around 55%.
Flow cytometry is often used to determine the levels of humanization by analyzing blood samples, with both the percentages and absolute number of cells quantified. Only mice with a humanization rate above a certain threshold, such as >25%, are considered suitable for experimentation.
If you want additional reassurance of your mouse model quality, it may be worth seeking a partner — a tailored relationship built on mutual trust — over simply a supplier. A partner can include additional checks for humanization, such as satellite mice that are sacrificed alongside the course of the experiment to assess the presence of human immune cells in secondary organs.
Mice with additional enhancements should also undergo further checks to ensure they are reliable. Boosted mice can be characterized by blood sampling one week after the boost to check for the presence of cells of interest, without requiring the sacrifice of the animal. These techniques allow for the accurate assessment of the quality of HIS mouse models.
Improve treatments with advanced HIS mouse models
An accurate pre-clinical model is vital to improve the likelihood of successful treatments for pathologies in which the immune system plays a role. HIS mouse models offer several advantages, including the ability to study the detailed interactions of immune system cells, and mice that possess stable immune systems over their entire lifetime. However, it is necessary to consider the way in which HIS mice are generated, as those generated through chemoablation have higher stability and improved welfare.
To be confident in the quality of your HIS mouse model, work with a partner who performs multiple checks including flow cytometry and immunohistochemistry. For more information, be sure to watch our webinar on using advanced human immune system humanized models in inflammatory disease research.
If you have questions or need more information about humanized immune system models and their applications, please contact us.

