- Blog
07/08/2025

Drug-induced liver injury (DILI) is a major public health issue, representing both a life-threatening risk for patients and a major cause of drug attrition at every step of its lifecycle, from preclinical development to Phase IV.
DILI is a diagnosis of exclusion, and symptoms, that can appear with variable latency, are akin to other conditions such as viral hepatitis, allergies, Metabolic-Associated Fatty Liver Disease or autoimmune hepatitis1. Three main types of hepatotoxicity manifestations can be described, based on liver biochemical profile: acute hepatitis, cholestasis, and mixed2,3. DILI can be classified as either dose-dependent (intrinsic) or not dose-dependent (idiosyncratic, iDILI) depending on the underlying etiology. iDILI can further be subclassified as immune-mediated (allergic) or non-immune-mediated (non-allergic), the second being the result of cellular damage caused by metabolite accumulation in hepatocytes2. These lesions originate from different pathophysiological mechanisms and their onset depends on genetic and environmental factors, as well as individual susceptibility.
Besides acetaminophen, the most famous inducer of dose-dependent hepatotoxicity, the most common medications known to trigger DILI are drugs targeting the central nervous system and antibiotics2. However, a wide diversity of more than 1 100 identified agents can induce DILI, including more than 100 herbal medicines and dietary supplements4, which are incriminated in around 16% of cases5. More recently, the rise of immune checkpoint inhibitors has led to the emergence of an increasing number of related cases of iDILI1.
Although the majority of patients can recover from DILI within 6 months after withdrawal of the suspected agent, around 30% will further develop chronic liver disease, undergo a transplantation, or die1,6.
Predicting DILI is therefore of paramount importance in the development of therapies.
The preclinical evaluation of the safety of novel therapies is a complex topic to address. Interspecies differences in hepatic metabolism may indeed result in both lack of efficacy and hepatic toxicity of the drug candidates in the clinical phases, despite good profile in animal models. The fact that DILI is still the root cause of 30% of pharmaceutical compounds attrition highlights the need for more predictive models.
Generally, dose-dependent toxicity is well predicted by preclinical studies, and incriminated drug candidates do not reach clinical Phases. In contrast, iDILI is far less predictable, because of its multi-faceted etiology, its dependence on individual and environmental characteristics, the variable latency before symptoms onset (weeks to months), and the dramatically small percentage of individuals affected7. The anticipation of its occurrence therefore represents the main challenge in innovative therapies development and a major pitfall in clinical development.
iDILI cases result from the synthesis of reactive chemical moieties during the metabolization of therapeutic agents. These byproducts activate the immune system by acting as neo-antigens, after forming adducts with proteins (haptenization)5. Because cytochrome P450 (CYP) enzymatic activity plays the leading role in drug metabolism, evaluating the metabolization of candidate medicines by this enzyme family is critical. In this context, the main limitations of animal testing arise from the interspecies differences in CYP enzyme-mediated metabolism.
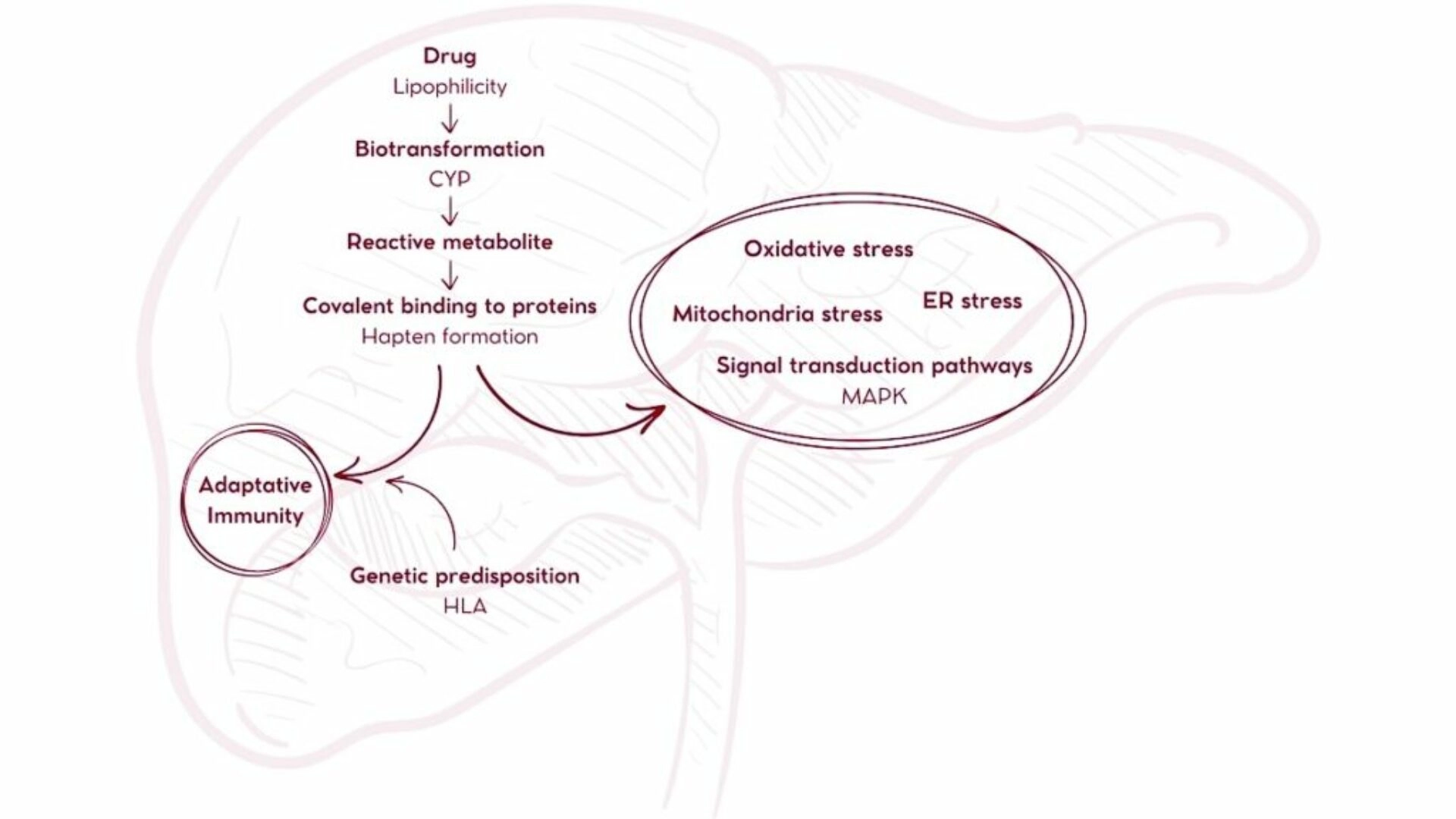
The production of reactive metabolites by CYP-mediated metabolization of the drug plays the central role in drug-induced liver toxicity
To overcome these limitations, primary human hepatocytes would stand as a model of choice, for they display the actual human metabolic pathways and CYP activity. However, the use of these cells is hampered by their short lifespan and the rapid loss of function and transcriptome profile alterations when cultured8, combined with a high cost and limited supply. The most recent advances in cell culture, however, partially mitigate these limitations9. Consequently, in vitro models classically rely on immortalized hepatic cell lines, such as HepaRG or HepG2, for early assessment of hepatotoxicity. These cancerous cell lines do not however faithfully recapitulate the physiology and metabolism of the healthy human liver.
Moreover, the byproducts may exert systemic effects that cannot be anticipated in vitro, and the pharmacodynamics, including route of administration, absorption, tissue-specific enzymatic activity and renal clearance, also influence the biological effects of a drug and consequently the occurrence of adverse events. An in vivo preclinical testing is therefore still necessary.
For these studies, Non-Human Primates would represent a model of choice, as they are the closest animal model to Humans, however, interspecies differences remain, associated with major ethical concerns regarding their use and excessive costs. On the other side, rodents are the most convenient model, for they are easily manageable and have a moderate cost, but the interspecies differences represent major issues.
The challenge of these interspecies differences lies in the dual risk of failing to detect toxicity in humans and mistakenly identifying an agent as toxic based on animal models when it is not harmful to humans. Two famous illustrations of these dual scenarios are furosemide and fialuridine (FIAU). FIAU is the typical example of the worst-case scenario in drug development. After successful preclinical and Phase I studies, Phase II produced tragic outcomes, as patients suffered from unexpected and severe hepatotoxicity, among other manifestations. Out of the 15 participants, 5 died and 2 more required liver transplantation10. In sharp contrast, the administration of furosemide in mice induces liver necrosis, while such adverse effect is not found in patients. After more than 50 years on the market, this agent has proven its safety in the clinical setting. The fact that testing a drug such as furosemide in conventional mice could have produced a false positive for hepatotoxicity and prevented the patient from accessing a safe and potent therapy for life-threatening conditions, such as heart failure, illustrates another aspect of the pitfalls of relying on preclinical findings and transposing them into the clinics.
Interestingly, those two iconic examples were tested in chimeric mice with humanized livers, producing results consistent with the clinics11,12.
Furosemide toxicity in mice is the result of the production by CYP enzymes of an epoxide metabolite, γ-ketocarboxylic acid. In a study comparing humanized-liver TK-NOG mice to non-humanized mice, the amount of this toxic metabolite in the liver and bile of control mice was found to be dramatically increased, together with poor survival and increased ALT11. In contrast, the evaluation of FIAU in the same chimeric mouse model revealed toxicity restrained to humanized-liver mice alone, the animal displaying increased serum ALT and lactate, lethargy, jaundice, and mortality, while control mice showed good tolerance. Further analysis confirmed that the toxicity of FIAU was restricted to human hepatocytes alone in chimeric livers, these cells displaying mitochondrial damage and lipid accumulation12.
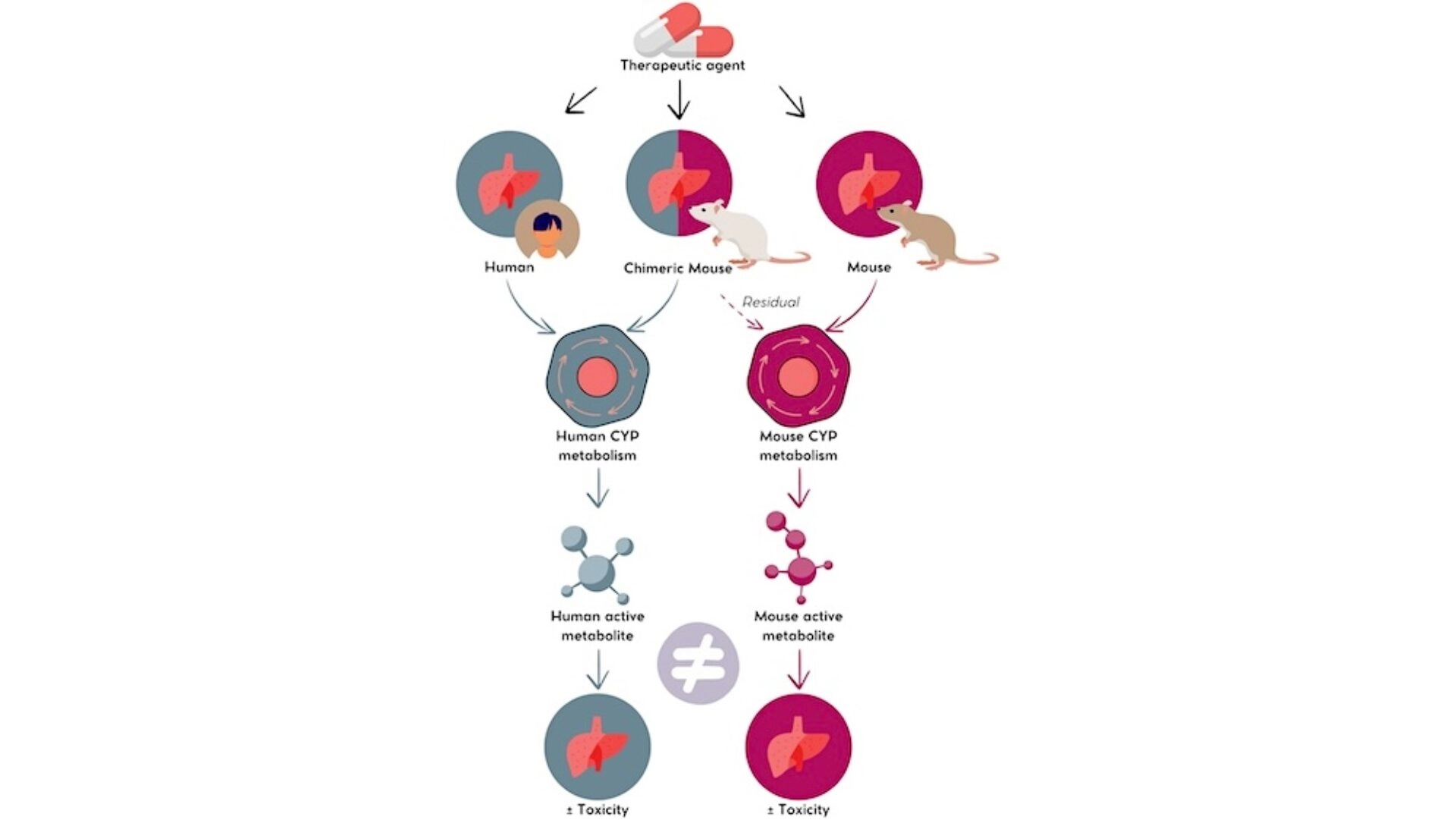
A given therapeutic agent may induce varying degrees of hepatotoxicity in human and murine livers due to species-specific CYP enzymatic activity, which produces different metabolites with distinct biological activities. The humanized-liver chimeric mouse model allows a better prediction of hepatotoxicity hazard
Besides these examples, the value of humanized mice in the preclinical evaluation of DILI was also reported in FRG, PIRF and PXB® strains with other therapeutic agents such as troglitazone13–15. In these models, the Human part of the chimeric livers displays the CYP enzymatic activity allowing to obtain a human-like metabolite profile and biological activity, although it must be noted that a residual murine activity can be detected14–17. The chimeric mouse with humanized liver therefore allows to see the bigger picture, offering a more complete, reliable, and predictive model, one step closer to drug safety.
To take it a step further, given the role of immunity in the pathophysiological mechanisms of DILI, the preclinical testing of drug candidates in double-humanized mouse models that support both human hepatocytes and the immune system would provide researchers with an even more comprehensive tool. In the context of the blooming field of immunotherapies in clinical settings, evaluating these cutting-edge therapeutics in double-humanized mice would offer profound insights into the interplay between immune cell populations and hepatocytes in this specific context.
These advanced preclinical models thus offer a highly valuable platform for the in vivo study of human liver drug metabolism before stepping into clinical Phases. Besides the prediction of iDILI, such models could also enable to improve DILI management and fulfill the current unmet medical need for specific antidotes.