- Blog
17/02/2025


The development of curative therapies is a crucial issue for the future management of Non-Alcoholic Liver Disease (NAFLD), and the success of this endeavor is highly dependent of our propensity to gain a deeper understanding of this pathology.
In the previous article, we explained how NAFLD is a major health burden, with an increasing incidence worldwide. We also discussed how inflammation and fibrosis progression are key targets in the search for innovant therapies, as they are major determinants of liver-related outcomes and overall mortality risk for patients.
This second blog explores the metabolic aspects of this disease, depicts the pitfalls faced by the drug candidates currently under development and give you some tips on how you can navigate through it by choosing the right preclinical mouse models to test innovative therapies.
Modelling NAFLD – The central role of liver metabolism
NAFLD is a progressive disease that encompasses a spectrum of several stages of liver damage, ranging from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), cirrhosis and ultimately hepatocarcinoma (HCC).
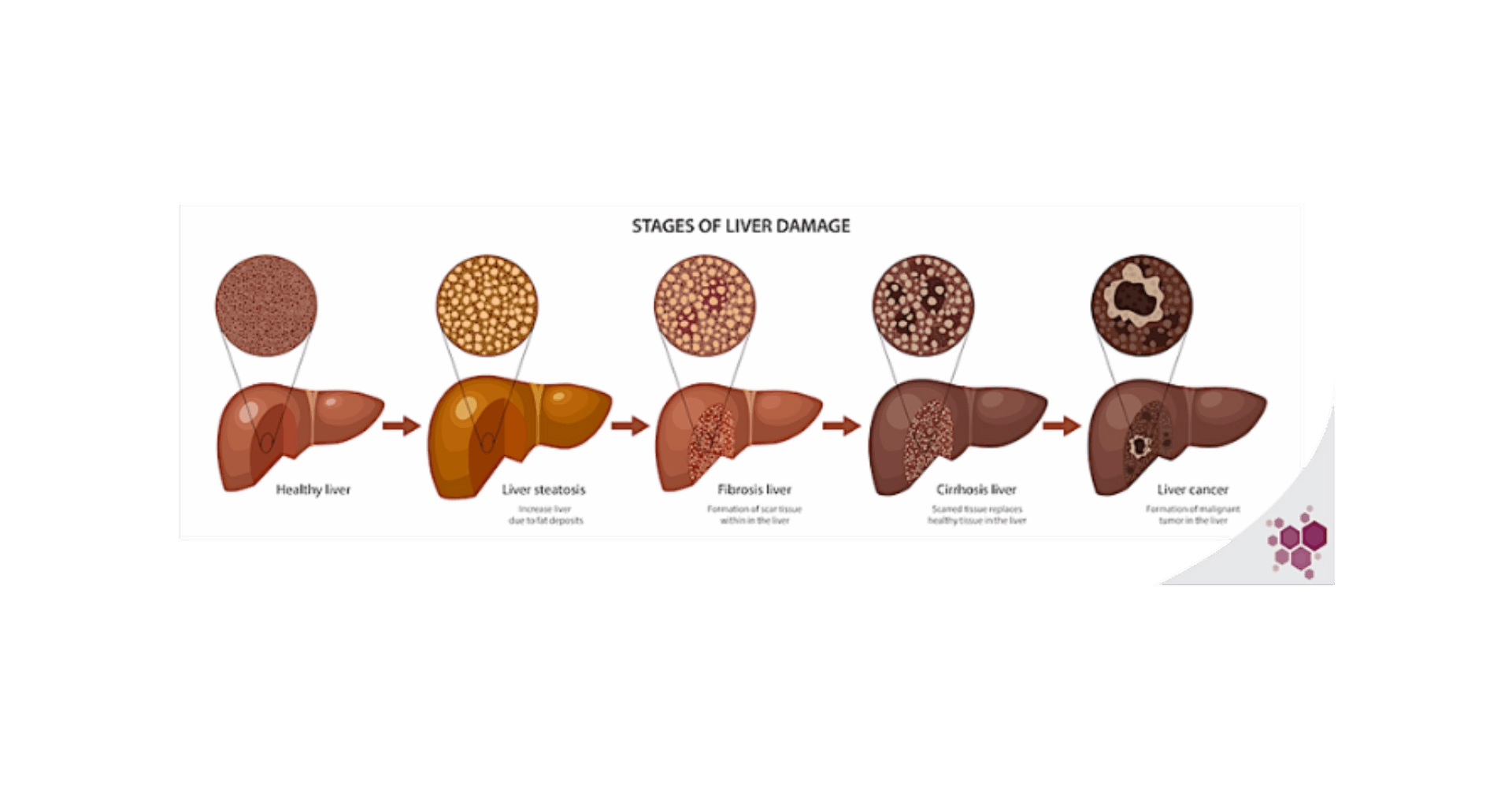
The early stages of liver lesions are reversible, until advanced fibrosis settles in late-stage NASH. The first triggers of these initial damages are the metabolic disturbances, many of which occur in the liver. In the most common clinical set up, these metabolic impairments originate from chronic over-nutrition, that leads to saturation of classical storage & metabolic processes, promoting ectopic fat deposition, adipokines release by the adipose tissue, mitochondrial dysfunction, and the synthesis of toxic metabolic byproducts, such as lipotoxic species (ceramides, free cholesterol…). In the liver, these phenomena result in the development of insulin resistance, oxidative stress, hepatocyte death, and activation of inflammation pathways as well as key drivers of fibrosis progression, such as hepatic stellate cells1,2. The multiplicity of metabolic processes involved in NAFLD progression offers just as many therapeutic targets, but the wide range of downstream functions regulated by metabolic pathways players, which are not yet fully described, also greatly complexifies the prediction of a drug’s success and may give rise to unexpected and severe side-effects. There is then a great need for preclinical models recapitulating as faithfully as possible the human liver metabolism.
Liver metabolism as therapeutic target
Several candidate therapies targeting the liver have been developed and have reached the clinical phases after encouraging preclinical results. However, to date, none has successfully passed the clinical trials and obtained the authorities approval. At the present time, one of the drugs candidates that went closest to reach the market is the steroidal farnesoid X receptor (FXR) agonist obeticholic acid (OCA). The FXR receptor is a major target in NAFLD as a central regulator of liver lipid and glucose metabolism, inflammation, liver regeneration and carcinogenesis. However, the benefit-risk balance proved unfavorable to OCA, as severe side-effect, such as hepatotoxicity, were reported in treated patients, despite a positive, albeit modest, effect on liver fibrosis3. Another serious candidate was elafibranor, a peroxisome proliferator-activated receptor (PPAR) α/δ agonist. PPARs are a family of nuclear receptors that play a central role in lipid and glucose metabolism and therefore represent a major target for metabolic diseases. Several drugs aiming at the three existing isoforms of this family (α, γ and δ) have been proposed, clinical trials showed nonetheless modest results in NASH patients and failed to meet primary endpoints4. However, the pan-PPAR agonist lanafibranor, currently in a Phase III trial (NCT04849728), could represent a new departure for this therapeutic class. Another strategy to hamper NAFLD progression is to boost liver fatty acid β-oxidation and cholesterol excretion trough the activation of the thyroid hormone receptor (THR). The drug candidate resmetirom, targeting the THR-β1 isoform, is currently under investigation, but according to the first disclosed results of the ongoing Phase III trial, primary endpoints are met at 52 weeks5. The sparsity of drugs targeting the liver metabolism to reach Phase III and their failure to meet the defined primary endpoints in most cases, despite encouraging results during the preclinical phases, highlight the complexity of addressing the liver intrinsic pathophysiological processes and the need to refine the preclinical models to give the most accurate picture of these mechanisms.
Targeting the metabolic disturbances is a self-evident approach to treating NAFLD.
However, the complexity of the processes linked to metabolic regulation, at both local and systemic levels, hinders the development of safe & effective therapies.
Choosing the right preclinical model to overcome the deadlocks in NAFLD drug development
No preclinical model can claim to provide an exhaustive and definite reflection of the complex and heterogenous physiopathology of human NAFLD, and for each scientific project, the appropriate model should be chosen carefully by thoroughly considering the scientific background of the study6. The targeting of hepatic metabolism dysfunction in NAFLD implies the choice of a model encapsulating the metabolic pathway of interest. Globally, the model of chronic overnutrition (e.g. western diet) recapitulates the most faithfully the NAFLD-associated metabolic disturbances, also allowing the development of obesity and insulin resistance. The two major limits of this model are 1) the long time required for the onset of marked NAFLD patterns and 2) the need for tightly controlled dietary fat and carbohydrates content to induce the lesions of interest. For this reason, the methionine- and/or choline-deficient diet (MCD) is often favored by researchers. These two nutrients are essentials for liver mitochondrial function and lipid metabolism7,8, and the MCD diet can lead to major intrahepatic lipid accumulation and the onset of typical histological NASH features as early as after 2 weeks on diet. However, this model is not fitted when addressing the systemic metabolic disturbances of human NAFLD. Similarly, chemically induced hepatic lesions models, such as carbon tetrachloride-induced hepatotoxicity, are relevant for the study of fibrosis but inappropriate for the study of NAFLD-associated metabolic disruptions. In contrast, genetically engineered mouse strains fed with a NASH-inducing diet can be useful to study specific pathways.
In addition to the delicate choice of a NAFLD model, the inherent shortcomings to the transposition of results from murine models to humans must be taken into account. Indeed, substantial discrepancy exist in liver gene expression patterns and metabolic pathways with respect to patients9. A classic example is lipoprotein profile. In humans, cholesterol is mainly carried in apolipoprotein B (apoB)-containing very-/low-density lipoproteins (VLDL/LDL), whereas in mice, high-density lipoproteins (HDL) are the main circulating form10. This difference is due to the lack of cholesteryl ester transfer protein (CETP) in mice, this enzyme being responsible for the transfer of cholesteryl esters from HDL to the apoB-containing lipoproteins11. This interspecies difference in cholesterol trafficking is of major importance, lipoprotein profile being a widely-used evaluation criterion in clinics, for low HDL cholesterol and high LDL cholesterol are historically associated with cardiovascular risk12. Besides these species-specific features, it must be kept in mind that in both mice and humans, the whole complexity of metabolic pathways is not yet fully elucidated. Fortunately, the development of humanized mouse models can pave the way to a deeper understanding of the metabolic dysfunctions involved and to a finer modeling of these.
At the preclinical level, the discrepancies between animal and human metabolic components and pathways add another layer of complexity that impedes the progress of research.
Modeling NAFLD in a humanized mouse model enables a more relevant and reliable preclinical evaluation of drug candidates, helping to anticipate with more accuracy the potential side-effects and thus reduce the risk of failure in clinical trials
A NAFLD model in the Hu-liver Mouse
The central role of liver metabolic disturbances in the development of NAFLD makes chimeric mice with humanized liver (Hu-liver) a valuable tool. Published studies have put to light the divergent transcriptomes of mouse and human hepatocytes in a chimeric liver exposed to a western diet13. Interestingly, in both the Hu-liver TIRF mouse and the Hu-liver FRGN mouse, the human moiety of the liver displayed the signature transcriptional profile of clinical NAFLD, highlighting the relevance of such preclinical models13,14. In these models, western diet feeding induced steatosis in human, but not murine, hepatocytes, suggesting a more pronounced sensitivity of human hepatocytes to diet-induced NAFLD13,14. In addition, in the FRGN chimeric mouse, typical NASH hepatic features were also reported after only 6 weeks of diet, with notably fibrosis development, inflammatory leukocytes infiltration and hepatocyte death, these lesions being not found in non-transplanted FRGN mice subjected to the same regimen14. Interestingly, Hu-liver mice present a human-like lipid profile on a regular diet15, which is altered by the western diet13,14, that also induces an increase in hepatic lipotoxic species content, such as ceramides and diacylglycerols13. It must be noted that similarly to what is observed in classical murine models, a great variability exists between strains in their propensity to develop NAFLD in response to dietary approaches. As an example, the Hu-liver cDNA-uPA/SCID (PXB®) mice are resistant to 12 weeks of western diet16, but do develop hepatic lesions (fibrosis, inflammatory cells infiltration, hepatocyte apoptosis) after 12 weeks of choline-deficient high-fat diet17. Interestingly, the PPARα/δ agonist elafibranor, which failed in Phase III, was tested in this latter model and produced results similar to those in the clinic17, emphasizing the value of Hu-liver NAFLD models for preclinical drug evaluation.
The development of such humanized models represents therefore a much-needed upgrade in preclinical research on NAFLD, not only providing a more accurate picture of the disease, but also enabling the testing of innovant therapeutics targeting liver metabolism, potentially saving time and resources.
Summary
Today, NAFLD represents a major public health challenge, and a specific pharmacological treatment remains an unmet medical need. Liver damage and metabolic disturbances resolution are key targets in the search for therapies, as they are major determinants of the progression to the more severe stages of the disease. However, the major interspecies differences between mice and humans in key metabolic components involved in NAFLD progression have been holding back research. In this context, the introduction of humanized mouse models represents an invaluable game-changer.
Considering the central role of inflammation in NAFLD pathophysiology, the major limitation of Hu-liver models is the immunodeficiency of the mouse strains used. A model of NAFLD in a dual liver and immune system humanized mouse would therefore stands as a top-of-the-art tool for therapies development.
Bibliography
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 15, 349–364 (2018).
Parthasarathy, G., Revelo, X. & Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatology Communications 4, 478–492 (2020).
May 19, 2023: Meeting of the Gastrointestinal Drugs Advisory Committee Meeting Announcement – 05/19/2023. FDA
Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis | Madrigal Pharmaceuticals.
Farrell, G. et al. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 69, 2241–2257 (2019).
Corbin, K. D. & Zeisel, S. H. Choline Metabolism Provides Novel Insights into Non-alcoholic Fatty Liver Disease and its Progression. Curr Opin Gastroenterol 28, 159–165 (2012).
Rinella, M. E. et al. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. Journal of Lipid Research 49, 1068–1076 (2008).
Teufel, A. et al. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues From Patients. Gastroenterology 151, 513-525.e0 (2016).
Camus, M. C., Chapman, M. J., Forgez, P. & Laplaud, P. M. Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. Journal of Lipid Research 24, 1210–1228 (1983).
Guyard-Dangremont, V., Desrumaux, C., Gambert, P., Lallemant, C. & Lagrost, L. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 120, 517–525 (1998).
Catapano, A. L. et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. European Heart Journal 37, 2999–3058 (2016).
Bissig-Choisat, B. et al. A human liver chimeric mouse model for non-alcoholic fatty liver disease. JHEP Rep 3, 100281 (2021).
Ma, J. et al. A Novel Humanized Model of NASH and Its Treatment With META4, A Potent Agonist of MET. Cell Mol Gastroenterol Hepatol 13, 565–582 (2021).
Ellis, E. C. S. et al. Mice with Chimeric Livers Are an Improved Model for Human Lipoprotein Metabolism. PLoS One 8, e78550 (2013).
Saxena, R., Nassiri, M., Yin, X.-M. & Morral, N. Insights from a high-fat diet fed mouse model with a humanized liver. PLoS One 17, e0268260 (2022).
Kisoh, K. et al. Estimating Drug Efficacy with a Diet-Induced NASH Model in Chimeric Mice with Humanized Livers. Biomedicines 9, 1647 (2021).