- Blog
22/05/2025


Non-alcoholic liver disease (NAFLD) is the most prevalent chronic liver disease worldwide, affecting more than a third of the global population and increasing rapidly, alongside the global epidemic of obesity and related metabolic diseases1.
The only effective treatment to date is weight loss and lifestyle habits changes, complemented by individually tailored pharmacological management of concomitant diseases and risk factors such as type 2 diabetes2,3. Alone, this approach is however insufficient to obtain satisfactory results and unfortunately, even if many drug candidates have reached the clinical phase, none has obtained marketing authorization.
NAFLD is a multifaceted condition, and non-alcoholic steatohepatitis (NASH) represents the initial critical stage leading to significant liver damages. Although NAFLD develops on a background of altered metabolism, NASH has a strong immunoinflammatory dimension promoting lobular inflammation2, cell damage and fibrosis4. NASH may eventually lead to cirrhosis and ultimately hepatocarcinoma (HCC), which is the second cause of liver transplantation after hepatitis C5.
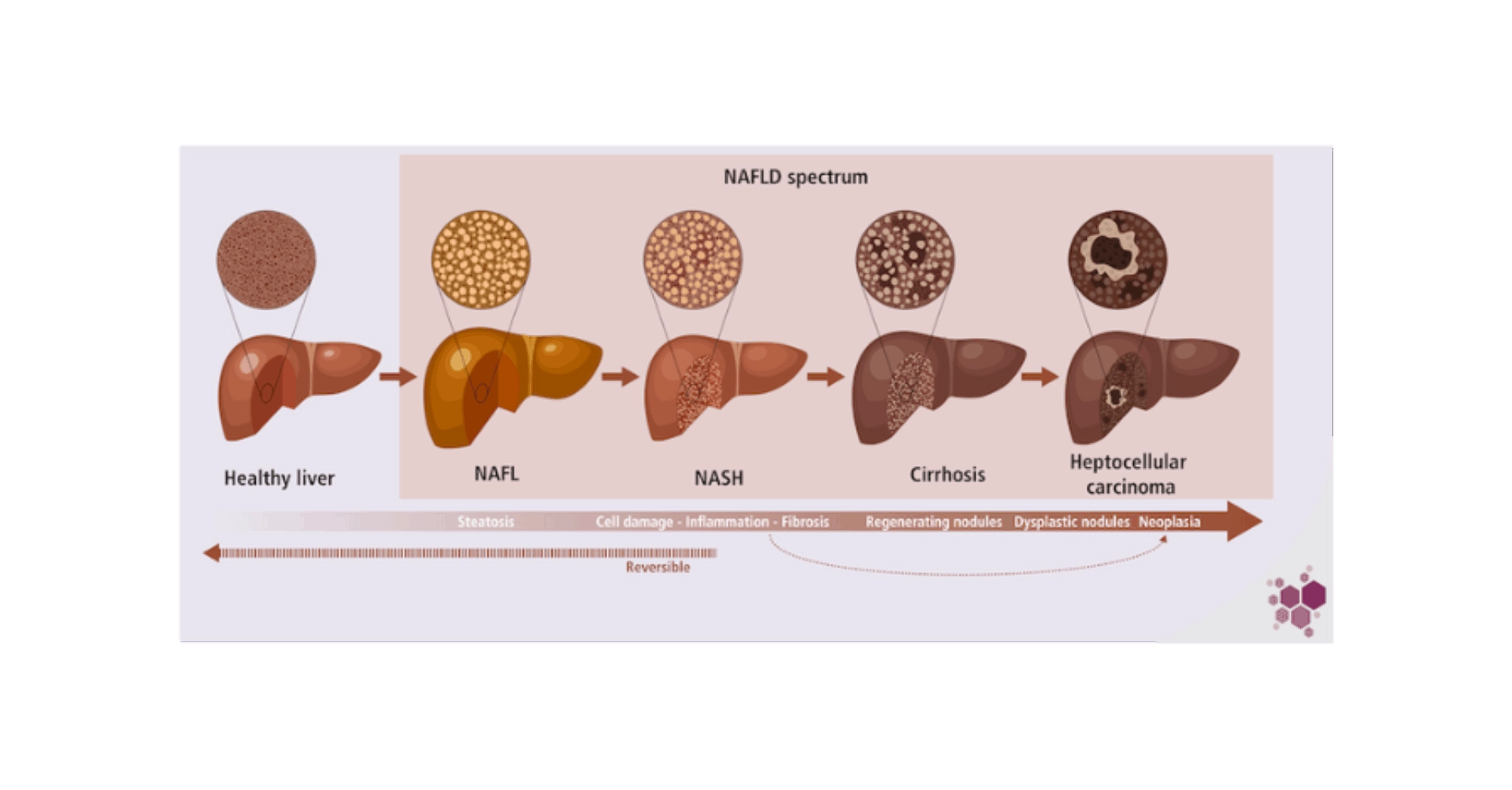
During NAFLD, and more particularly NASH, cell repertoire is reshaped, with significant alterations notably in the myeloid section, characterized by a marked increase in inflammatory monocytes and monocytes derived cells (monocyte derived Kupffer cells)6. This influx plays a role in the unrestrained inflammatory milieu that drives liver damage and advances NASH. The hepatic immune cell repertoire comprises also conventional CD8+ T cells and CD4+ T cell subsets, innate-like T cells, such as invariant natural killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells and γδ T cells, which participate also in the uncontrolled inflammatory environment promoting hepatocyte death and fibrosis, further exacerbating the condition. Neutrophils and dendritic cells (type 1 conventional DCs) accumulation is an early event in NASH that further promotes inflammation and liver injury. B cells and, in particular, IgA+ plasma cells also contribute to NASH and the progression to HCC through their immunosuppressive effects by inducing CD8+ T cell exhaustion7. Recent advances suggest that the immunomodulation of NASH is certainly a complex crosstalk between immune cells, hepatic cells and no parenchymal cells, including hepatic stellate cells (HSC) and liver sinusoidal endothelial cells7. The immune cells can promote inflammation directly, by secreting inflammatory cytokines, but also indirectly by activating neighboring immune and non-immune cells such as hepatocytes and HSC. However, the intricate nature of these interactions is just beginning to be unveiled and it is still unclear whether immune cells drive NASH by direct signaling to hepatocytes and HSC, or indirectly through a more complex interaction network. Emerging techniques and models are expected to enhance our grasp of the communication pathways at play during NASH in the foreseeable future.
There are currently no approved therapies for NASH treatment. Therapies targeting the immune-inflammatory branch have failed in clinical trials despite showing promising results in preclinical phases. This is the example of the promising Cenicriviroc (CVC) treatment, a dual CCR2 and CCR5 antagonist that is able to block CCR2/5 on macrophages, T cells and HSC, which are essential players in NASH. CVC demonstrated promising results during the Phase II trial, but efficacy did not meet expectations during the Phase III. Nevertheless, CVC is still investigated in combination strategies (e.g. tropifexor/TANDEM trial)8. Similarly, the humanized monoclonal antibody Simtuzumab sparked optimism for fibrosis therapy but failed to show antifibrotic effects in NASH patients with bridging fibrosis or compensated cirrhosis8. Pentoxifylline has not been reported to specifically affect macrophages, but it attenuates TNF release and oxidative stress, and has shown significant improvement in histological steatosis, inflammation, hepatocyte ballooning and fibrosis. Pentoxifylline is currently in Phase III trial evaluation9. Recently, alternative therapeutic options like cell therapy approach have been highlighted to limit, notably, fibrosis in mouse models. This is the case of the development of CAR-T (chimeric antigen receptor T-cells) that target uPAR+ (urokinase-tupe plasminogen activator receptor) HSC and macrophages, which reduces fibrosis and improves liver functions10. However, these new therapies have yet to successfully pass the clinical trial phases.
At the preclinical level, the hurdles in addressing the NAFLD therapeutic niche mostly arises from the difficulty of accurately accounting for the complexity and the multifactorial nature of the pathophysiological mechanisms involved in disease progression.
This systemic interplay between metabolic disturbances and inflammatory pathways activation hinders the development of safe & effective therapies.
No preclinical model can fully capture the complex and heterogenous physiopathology of human NAFLD, and for each scientific project, the appropriate model should be chosen carefully by thoroughly considering the scientific background of the study11. Animal models of NAFLD includes:
Dietary models:
→ The chronic overnutrition (CO) model, that faithfully replicates the pathophysiological process involved in NAFLD and also allows the development of obesity and insulin resistance.
→ The short-duration methionine- and/or choline-deficient (MCD) diets, that allows the acute development of hepatic steatosis and fibrotic lesions.
Genetically engineered mouse strains fed with NASH-inducing diet, useful to study specific pathways.
Chemical models (carbon tetrachloride-induced hepatotoxicity), used to prompt significant liver fibrosis.
This variety of available models underline the struggles met when designing a reliable and accurate preclinical model of NAFLD. In addition to this, are the inherent shortcomings to the transposition of results from murine models to humans. Indeed, murine NAFLD-like liver pathology induced by dietary models provides valuable insights into the development of NAFLD, nevertheless, there are still substantial discrepancy in liver gene expression patterns with respect to NAFLD patients12, and the role of different human immune subsets in NAFLD pathogenesis is not comprehensively defined. Fortunately, the development of humanized mouse models can help to overcome these limitations.
Interspecies differences limit the transposability of results between rodents and humans.
Modeling NASH in a humanized mouse model enable more relevant and reliable preclinical evaluation of candidate therapies, helping to reduce the risk of failure in future clinical trials.
Depending on the target of interest, a variety of NASH models are available.
The central role of systemic inflammatory processes in the development of NAFLD, notably by promoting fibrosis progression13, makes chimeric mice with humanized immune systems a valuable tool as well in the search for novel NAFLD therapies. Indeed, studies conducted in humanized mouse models have put to light the central role of specific subsets, such as CD4+ T cells, in the development of the disease14. The use of such humanized model therefore represents a much-needed upgrade in preclinical research on NAFLD, not only providing a more accurate picture of the disease, but also enabling the testing of innovant therapeutics targeting inflammation and fibrosis, potentially saving time and resources.
Models of NASH in humanized mice can be established using CO or MCD diets in CD34+ humanized animals. They offer distinct advantages, such as:
Development of a complete and functional human immune system: CD34+ humanized mice support the development not only of T cells, but also B cells, NK cells, dendritic cells, monocytes and macrophages, allowing testing of drugs with various mechanisms of action and targets, including T-cell engagers, macrophage polarization agents and anti-fibrotic drugs
Infiltration of all mouse tissues, including the liver, with human immune cells
Development of not only steatosis, but also features of human NASH, like strong inflammation and fibrosis
Correlation between serum fibrotic markers and CD68 liver infiltration
The possibility of quantifying human serum cytokines : IFNg, IL-17A , TNF, IL-6, IL-8, IL-10, IL-18, MCP-1…
NAFLD is a major health burden, and its incidence is increasing worldwide. Inflammation and fibrosis progression are key targets in the search for therapies, as they are major determinants of liver-related outcomes and overall mortality risk for patients. In this context, understanding the physiopathology under the disease will enable us to develop alternative therapeutic option that would be testing in the most appropriated mouse model in order provide the most accurate results before clinical trials.
In our next blog, we’ll delve deeper into the metabolic arm of the disease and how you can navigate through it by choosing the right preclinical mouse models.
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335 (2023).
EASL, E. A. for the S. of the L., EASD, E. A. for the S. of D. & EASO, E. A. for the S. of O. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology 64, 1388–1402 (2016).
Cusi, K. et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocrine Practice 28, 528–562 (2022).
Angulo, P. et al. Liver Fibrosis, but no Other Histologic Features, Associates with Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 149, 389-397.e10 (2015).
Younossi, Z. M. et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clinical Gastroenterology and Hepatology 17, 748-755.e3 (2019).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Huby, T. & Gautier, E. L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol 22, 429–443 (2022).
Trauner, M. & Fuchs, C. D. Novel therapeutic targets for cholestatic and fatty liver disease. Gut 71, 194–209 (2022).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 16, 145–159 (2019).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Farrell, G. et al. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 69, 2241–2257 (2019).
Teufel, A. et al. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues From Patients. Gastroenterology 151, 513-525.e0 (2016).
Nati, M., Chung, K.-J. & Chavakis, T. The Role of Innate Immune Cells in Nonalcoholic Fatty Liver Disease. J Innate Immun 14, 31–41 (2021).
Her, Z. et al. CD4+ T Cells Mediate the Development of Liver Fibrosis in High Fat Diet-Induced NAFLD in Humanized Mice. Front Immunol 11, 580968 (2020).