- Blog
17/02/2025

Cytokine Release Syndrome (CRS) represents a significant challenge in the realm of immunotherapy, characterized by an overwhelming release of pro-inflammatory cytokines leading to severe systemic inflammation and potential organ dysfunction (Figure 1 below). Understanding the multifaceted nature of CRS is crucial for developing safe and effective therapeutic strategies.
Figure 1. Cytokine Release Syndrome (CRS) manifests as an uncontrolled systemic inflammatory response, characterized by the excessive release of pro-inflammatory cytokines. This overwhelming immune reaction can lead to widespread organ dysfunction and failure, highlighting the critical nature of CRS in affected patients. This figure provides a concise depiction of the syndrome’s impact on systemic health.
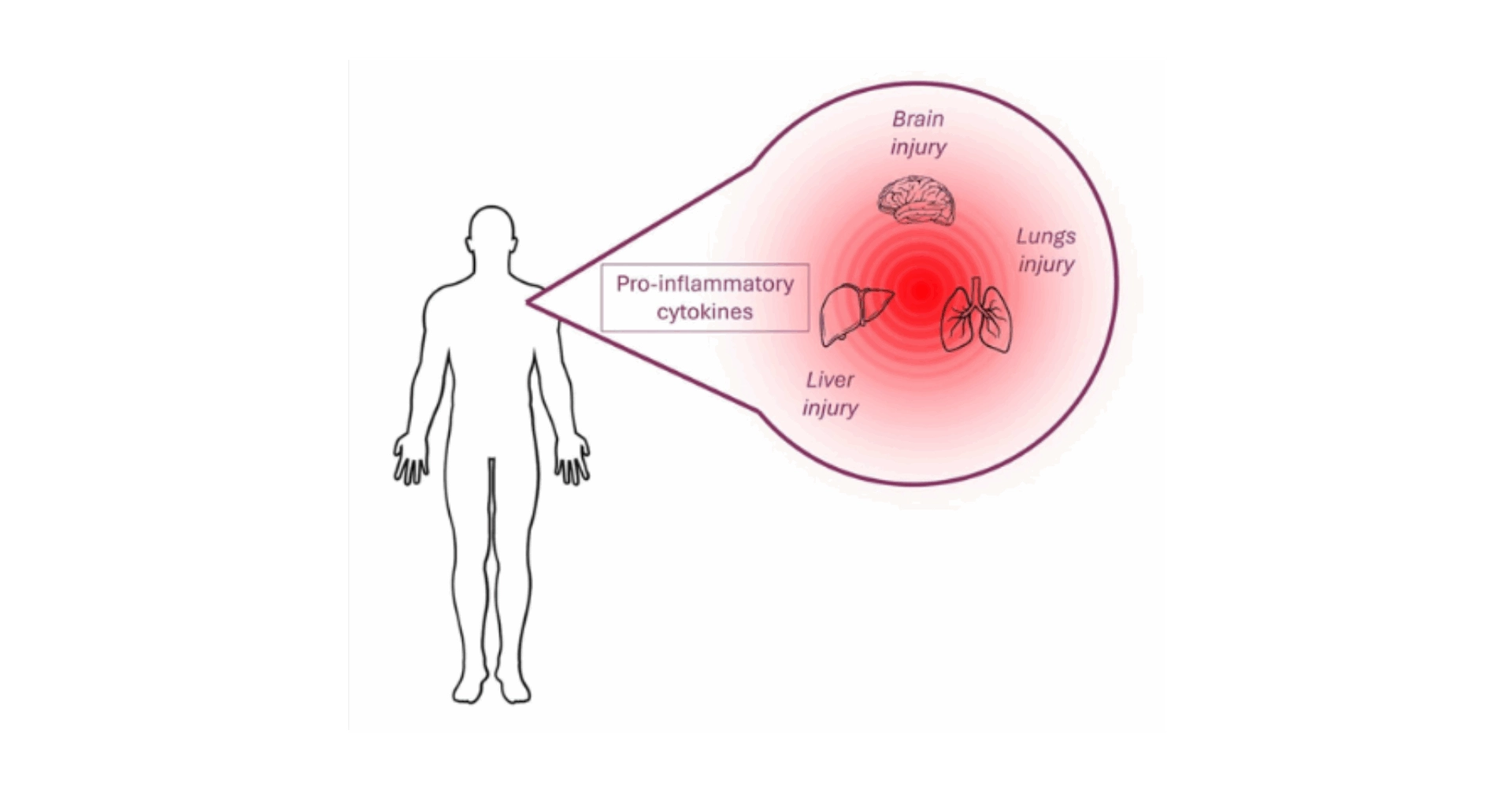
CRS represents a critical immune-mediated condition characterized by an uncontrolled release of pro-inflammatory cytokines and chemokines, including IL-1, IL-2, IL-6, IL-10, CXCL8, CXCL10, CCL2, CCL3, CCL4, IFN-γ, TNF-α, and GM-CSF. This hyperinflammatory state can be triggered by a variety of factors, such as (i) immune or tumor cells (autoimmune diseases or cancer), (ii) viral infections (notably Influenza and COVID-19), and (iii) the administration of immunotherapies (Figure 2 below). These therapies include monoclonal antibodies targeting immune cell markers (such as CD3, CD28, CD52, CD20, CD30, CD40, PD-1, CTLA-4), bi-specific antibodies (CD3-CD19), and CAR (Chimeric Antigen Receptor) T cell therapies. CRS manifestations vary widely, from mild symptoms like fever and headaches to severe, life-threatening conditions such as respiratory distress syndrome and multi-organ failure, underscoring the syndrome’s potential to significantly impact patient health and treatment outcome1–5.
Figure 2. The Multifaceted Pathophysiology of Cytokine Release Syndrome. Various triggers, including viral infections, autoimmune diseases, and immunotherapeutic agents, initiate the stimulation of immune cells. This activation results in the extensive release of cytokines, driving systemic inflammation that can lead to a spectrum of symptoms ranging from mild to severe, ultimately risking multi-organ failure.
Addressing Cytokine Release Syndrome (CRS) necessitates a sophisticated, multi-layered strategy targeting the complex inflammatory pathways involved in its development. Central to CRS is a dysregulated immune response, predominantly driven by T lymphocytes. Their continuous activation or destruction by internal or external stimuli triggers the release of IFN-γ and TNF-α, leading to cellular death and initiating a secondary inflammatory loop. This loop involves the recruitment and activation of the myeloid compartment, resulting in an overproduction of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ, which in turn causes widespread inflammation and potential organ damage. Consequently, CRS manifests as an amplified and abnormal inflammatory response without resolution, deviating from the path to homeostasis. This hyperactivation of the immune system can be triggered by a variety of factors, not limited to immunotherapeutic agents aimed at enhancing immune activity against cancer cells or infectious agents. Early management strategies typically focus on utilizing cytokine inhibitors to reduce the inflammatory response. Agents like Anakinra, which blocks IL-1, and Tocilizumab, targeting IL-6R, are frequently used in conjunction with corticosteroids, renowned for their extensive anti-inflammatory effects. In more severe cases, the implementation of supportive care measures, such as supplemental oxygen and vasopressors, is critical6–8.
The heterogeneous nature of CRS, influenced by its varied causes and complex pathophysiology, highlights the necessity for tailored therapeutic approaches that are both precise and patient-specific.
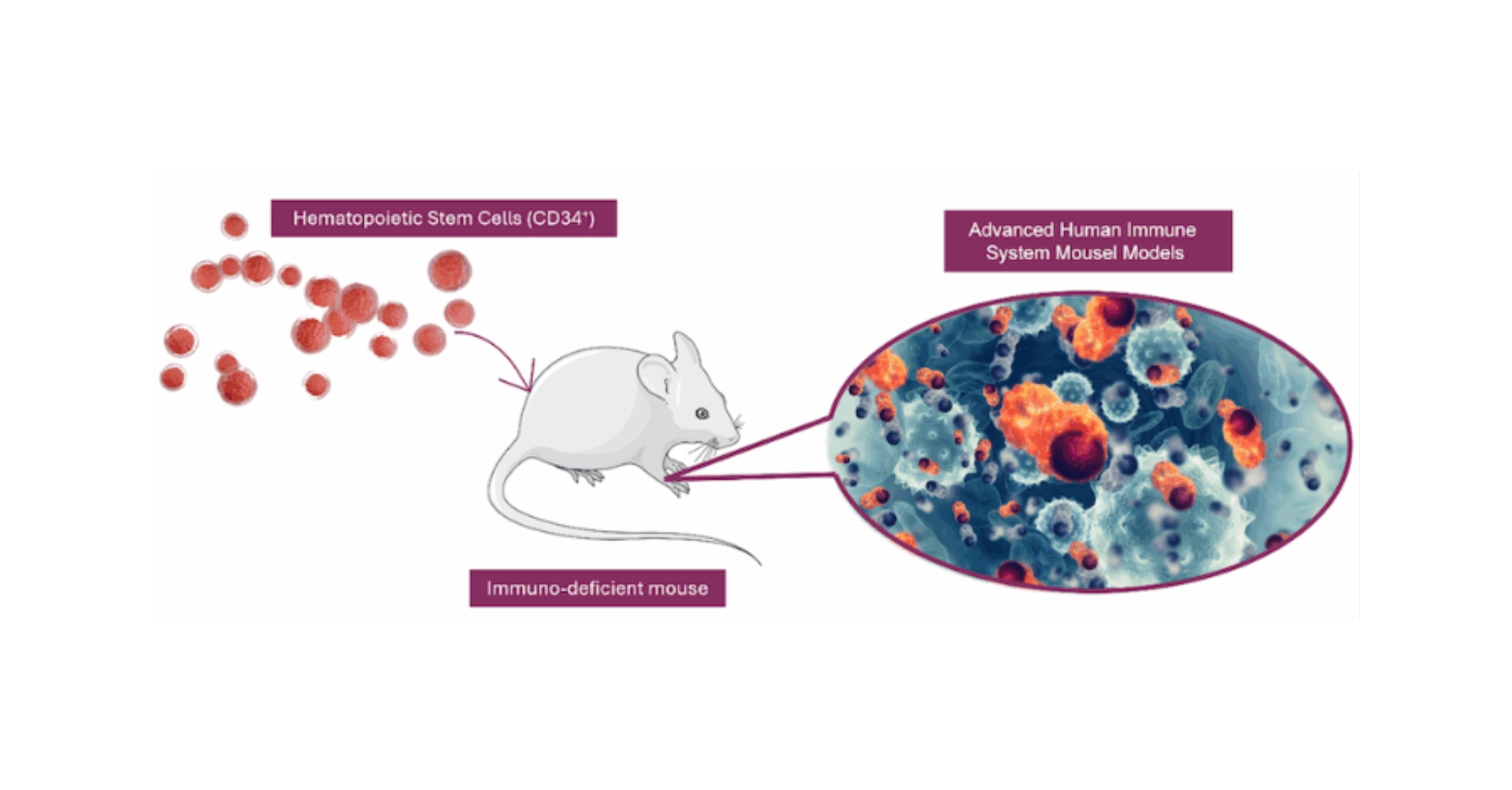
In the pursuit of unraveling the intricacies of Cytokine Release Syndrome (CRS) and advancing the development of cutting-edge immunotherapies, researchers have increasingly turned to humanized mouse models, especially those engrafted with CD34+ hematopoietic stem cells (HSC). These advanced models are pivotal in dissecting the human immune system’s nuanced response to CRS, shedding light on the intricate interplay between lymphoid and myeloid cells. Unlike traditional syngeneic or immunodeficient mouse models, CD34+ humanized mice offer a more accurate simulation of human immune responses, proving essential for the comprehensive study of CRS’s initiation, progression, and potential mitigation strategies. The incorporation of CD34+ HSCs into these models facilitates the development of a functional human immune system within the mouse, encompassing a broad spectrum of immune cells, including T cells, B cells, NK cells, monocytes, and dendritic cells (Figure 3).
Figure 3. Comprehensive Human Immune System Reconstitution in CD34-Humanized Mice. Severely immunodeficient mice are engrafted with CD34+ hematopoietic stem cells derived from human umbilical cord blood, leading to the development of a fully functional human immune system within the mouse model. This system encompasses a wide array of immune cells, including monocytes, dendritic cells, natural killer (NK) cells, T cells, and B cells, mirroring the complexity and diversity of the human immune response.
This diversity allows for a detailed examination of the immune cascade triggered by CRS, from the initial cytokine storm to the subsequent immune cell activation and interaction. The presence of human-like myeloid and dendritic cells in these models is particularly crucial, as these cells play significant roles in antigen presentation, cytokine production, and the modulation of T cell responses, which are central to the pathogenesis of CRS. Moreover, the CD34+ humanized mouse model enables the study of the effects of immunotherapeutic agents on human immune cells in a controlled environment. This is vital for assessing the potential risks of CRS associated with new therapies and for developing strategies to mitigate these risks. By closely mimicking the human immune response, these models provide invaluable insights into the mechanisms driving CRS and the effectiveness of interventions designed to prevent or alleviate the syndrome. The scientific advancements facilitated by CD34+ humanized mice extend beyond CRS, offering a versatile platform for exploring the immune system’s complexities and the impact of various therapeutic agents9–15.
As such, these models represent a significant leap forward in our ability to understand and combat the challenges posed by CRS, paving the way for safer and more effective immunotherapeutic strategies.
Discover how TransCure bioServices can elevate your CRS research. With our cutting-edge CD34-NCG humanized mouse models and deep expertise in immunotherapy evaluation, we’re ready to support your next breakthrough. Our optimized CD34-Humanized mice display a full human immune system including a fully functional myeloid compartment, well described to play a fundamental part in the CRS pathogenesis. We boost the maturation of the innate immunity compartment via a transient expression of selected human cytokines using the hydrodynamic boost technique. This capability allows for an in-depth exploration of the mechanisms behind CRS, especially the involvement of myeloid cells in the inflammatory response.
Advancing CRS research necessitates a comprehensive understanding of its multifaceted nature, as it poses a significant challenge in immunotherapy due to the severe systemic inflammation and potential organ dysfunction caused by an excessive release of pro-inflammatory cytokines. The condition’s complexity is underscored by its triggers, which range from viral infections and autoimmune diseases to immunotherapeutic interventions, leading to a spectrum of symptoms that highlight the need for effective management strategies. Addressing CRS involves a nuanced approach, employing cytokine inhibitors and supportive care tailored to its varied causes and complex pathophysiology. The development and utilization of CD34+ humanized mice models have been pivotal in providing deeper insights into the human immune response to CRS, thereby aiding in the evaluation of emerging immunotherapies’ safety and efficacy. TransCure bioServices stands at the forefront of this research, offering cutting-edge humanized mouse models to support the advancement of CRS understanding and the development of innovative therapeutic solutions.