Hepatotoxicity refers to liver damage caused by chemical substances, including drug candidates, and remains a major cause of clinical trial failure and post-market drug withdrawal. Predicting human-specific liver toxicity in preclinical studies is challenging due to species differences in metabolism and hepatocyte sensitivity.
TransCure bioServices humanized liver mouse model – based on TK-NOG mice engrafted with primary human hepatocytes – offers a powerful platform for assessing human-specific hepatotoxic effects. The model supports long-term studies with stable human albumin levels and maintains functional human liver physiology, including expression of key enzymes and transporters involved in drug metabolism.
Main characteristics
-
TK-NOG Humanization
TK-NOG immunodeficient mice are pre-conditioned with ganciclovir, which activates the thymidine kinase (TK) transgene, selectively inducing apoptosis of mouse hepatocytes and creating space for human cell engraftment. This is followed by intrasplenic injection of healthy primary human hepatocytes, enabling efficient seeding and repopulation of the liver. Approximately 8 weeks post-engraftment, mice are quality controlled by measuring human albumin levels in the blood, which serves as a surrogate marker for the Replacement Index (RI)—a key indicator of the extent of human liver reconstitution.
-
Healthy animals
Unlike other humanized liver mouse models, humanized TK-NOG mice do not develop liver tumors for up to one year of age, allowing for a more stable and physiologically relevant liver environment. This extended tumor-free window enables longer-term studies and ensures that liver function and architecture remain closer to the human physiological state, enhancing the model’s translational value for drug metabolism, toxicity, and liver disease research.
-
Readouts
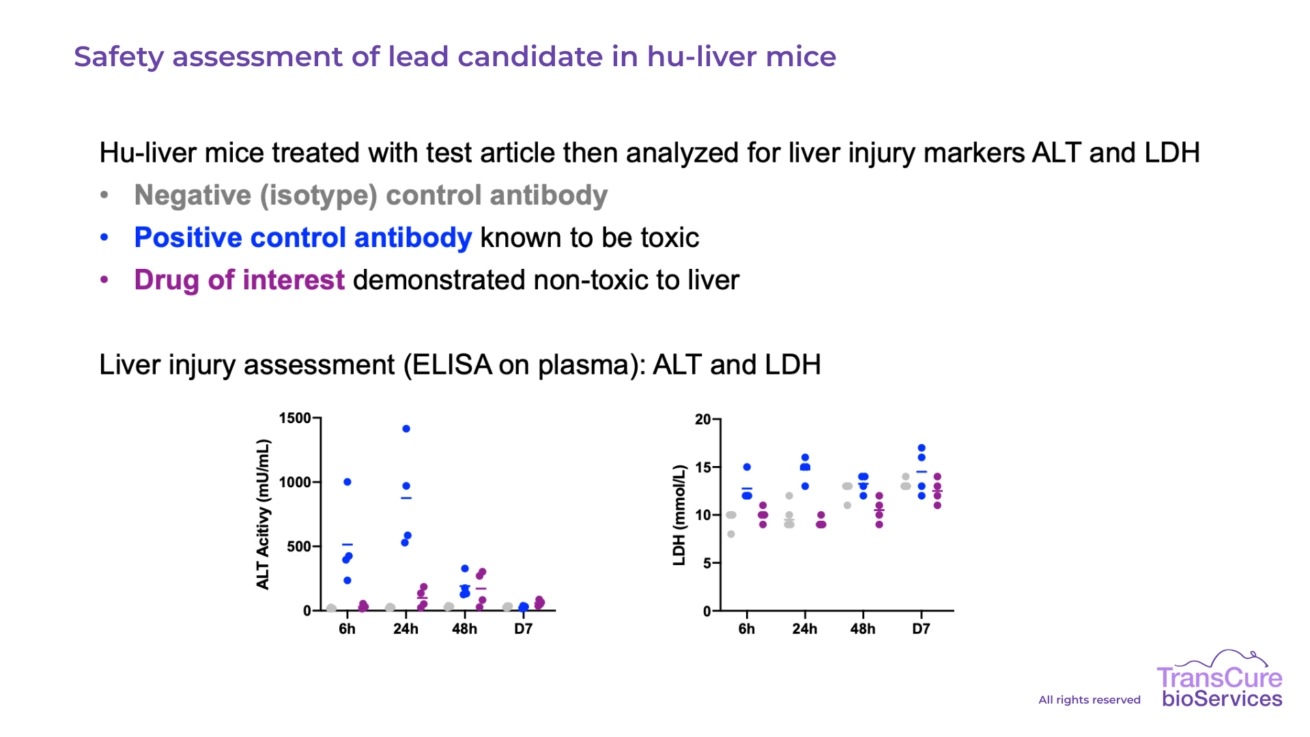
In our humanized liver mouse model, hepatotoxicity can be evaluated through several robust and clinically relevant readouts. These include measurement of serum biomarkers such as ALT, AST, bilirubin, and albumin to assess liver function, alongside histological analysis to detect hepatocellular damage, necrosis, or fibrosis. Additionally, immunohistochemistry (IHC) and gene expression analysis can further elucidate molecular mechanisms of drug-induced liver injury (DILI), ensuring comprehensive evaluation of hepatic safety in a fully human liver context.
-
Human Metabolite Production
Assessment of human-specific metabolite production is reliably achievable, facilitating accurate evaluation of drug metabolism pathways and pharmacokinetics. Frequent blood sampling allows detailed PK/PD profiling, including time-dependent measurement of drug concentration and metabolite generation, closely mirroring clinical conditions. This approach enables precise characterization of human hepatic drug processing, enhancing translational relevance and predictive accuracy of preclinical studies.
-
The Fialuridine Case Study
A landmark example is fialuridine (FIAU), an antiviral compound that passed standard preclinical tests but caused fatal liver failure in humans. In conventional animal models, FIAU appeared safe due to species-specific differences in mitochondrial toxicity. However, studies using humanized liver mice have successfully recapitulated FIAU’s mitochondrial toxicity and hepatotoxic profile, making this model a gold standard for assessing human-relevant liver safety and preventing late-stage failures.
Applications
The right mouse model for your research experimentation
-
Humanized Liver Mouse Model
Assess the efficacy and hepatotoxicity of your lead candidate in a clinically relevant human liver environment, established by engrafting primary human hepatocytes into immunodeficient mice.
See the mouse model -
Double Humanized Mouse Model
Combine a full human immune system with a chimeric human liver in the same mouse.
See the mouse model
Contact us to have more information about a mouse model
Contact usKnow everything about the CD34+ humanized mouse
-
Which routes of administration are feasible?
We offer a comprehensive range of administration routes tailored to your study requirements, including intravenous (IV), intraperitoneal (IP), subcutaneous (SC), oral (PO), intramuscular (IM), intratumoral (IT), intranasal (IN), intrarectal (IR), and intratracheal (ITR) delivery. Our skilled team can also adapt or develop specialized administration methods to align with your experimental needs. -
Do you provide mass spectrometry (MS) analysis?
While we do not perform mass spectrometry analysis in-house, we can collect and ship plasma or serum samples directly to your facility or to a laboratory of your choice for MS analysis.
You have more questions ?
If you have further questions or would like to discuss how our Humanized Liver Mouse Model can be tailored to your needs in Hepatotoxicity assesment, our scientific team is here to help. We’re committed to providing clear, responsive support and working closely with you to design studies that align with your objectives. Don’t hesitate to reach out - we’re always happy to share our expertise and explore solutions together.
Contact us