
Inflammatory Diseases: IBD mouse model
Inflammatory Bowel Disease in Human Immune System Mouse Model
Contact usInflammatory Bowel Disease (IBD) encompasses chronic inflammatory conditions of the gastrointestinal tract, primarily Crohn’s disease and ulcerative colitis. These disorders are characterized by an abnormal immune response to intestinal microbiota, leading to tissue damage, altered barrier function, and persistent inflammation.
TransCure bioServices offers chemically induced mouse models of IBD using DSS (Dextran Sulfate Sodium) or TNBS (2,4,6-Trinitrobenzenesulfonic acid) to trigger acute or chronic intestinal inflammation. These models reliably reproduce hallmark features of human IBD, including epithelial erosion, immune cell infiltration, and cytokine-driven inflammation.
Main characteristics
-
Global IBD Score
Throughout the study, mice are monitored daily or as needed to assess key clinical signs, including body weight loss, diarrhea, and rectal bleeding. These parameters are combined to calculate a comprehensive IBD clinical score, providing a consistent and quantitative measure of disease severity.
-
Endoscopy
Endoscopy offers valuable additional readouts in IBD studies by providing a detailed, real-time view of the colonic mucosa. It enables longitudinal monitoring of mucosal lesions, allowing for early and accurate detection of colitis, often before clinical scores reflect disease onset. Endoscopy also supports direct assessment of treatment efficacy over time and shows a strong correlation with histological findings, making it a powerful tool to complement clinical and tissue-based analyses.
-
Histopathology
as colon shortening is a hallmark of IBD-related inflammation. Tissues are then processed for histological analysis, typically using H&E staining, to assess and quantify key pathological features such as tissue damage, ulceration, and inflammatory cell infiltration. This provides a detailed and quantitative evaluation of disease severity at the tissue level.
-
Human Immune Cell Infiltration
The colon can also be analyzed to characterize and quantify human immune cell infiltration, either by flow cytometry following tissue dissociation or through immunohistochemistry (IHC). These complementary approaches provide detailed insights into immune cell composition, localization, and activation status within the inflamed tissue, enhancing the understanding of treatment effects on the human immune response.
-
DSS Model
The DSS model, frequently used to simulate ulcerative colitis, involves administering DSS in the drinking water . This exposure leads to damage to the colonic epithelial cell monolayer, triggering the invasion of bacteria and related antigens into the mucosa, which in turn induces the secretion of human pro-inflammatory cytokines such as IL-6 or TNF-alpha.
-
TNBS Model
The TNBS mouse model initiates colitis via the intra-rectal administration of TNBS, a hapten agent, dissolved in ethanol. This procedure, involving a single TNBS dose, triggers acute inflammation specifically in the distal portion of the colon. The critical role of CD4+ T cells bearing a Th1 profile in the onset of colitis underscores the relevance of this model to human conditions. The mechanism involves ethanol disrupting the epithelial barrier, which facilitates TNBS binding to mucosal proteins. This binding renders the proteins immunogenic, setting them up for processing by antigen-presenting cells in the lamina propria. The subsequent reaction triggers an excessive IL-12 secretion by macrophages, inducing a robust Th1-pro-inflammatory immune response.
-
Acute Model
The acute model of IBD follows a precise schedule. From day 1 to day 7, we introduce DSS solution to the mice’s drinking bottles. The period from day 8 to day 12 serves as a wash-out phase, where we replace the DSS solution with water. The termination of the acute model can be set between days 11 and 13 post-induction, subject to the health status of the animals. Typically, treatments start prior DSS administration.
-
Chronic Model
The protocol for the chronic IBD model involves three distinct cycles of DSS administration, each serving to perpetuate a chronic condition of IBD. The first cycle initiates on Day 1, with subsequent cycles commencing on Days 12 and 23. From Day 1 through Day 27, we provide the animals with the DSS solution in their water bottles. A wash-out period starts from Day 7 and extends to Day 33, during which we substitute the DSS solution with water until the cycle concludes. The chronic model can be terminated between Days 31 and 35, depending on the health of the animals and the client’s specific needs. Typically, treatments start a few days following DSS induction.
Applications
-
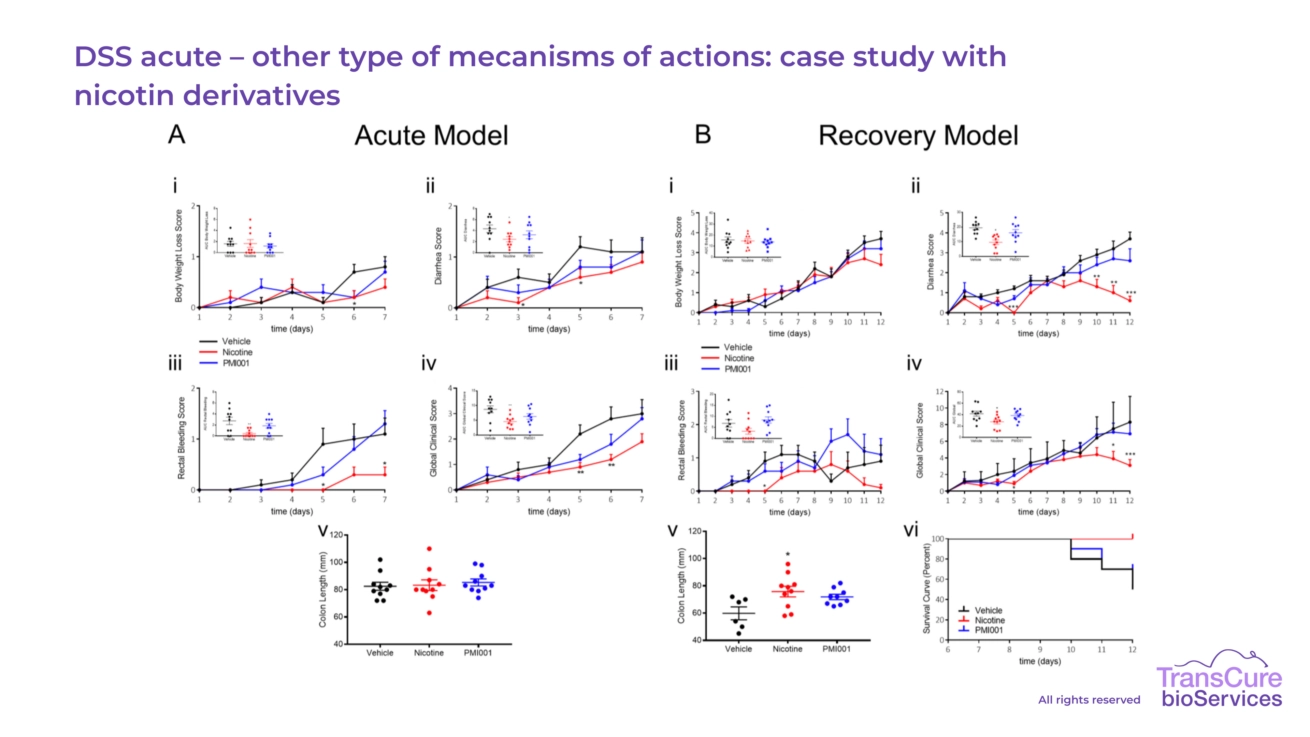
Our team’s contribution to a collaborative study with PMI, titled “Tobacco Alkaloid Assessment in a DSS-Induced Colitis Mouse Model with a Fully Humanized Immune System” and published in the International Journal of Molecular Sciences (March 2023), highlights the potential of tobacco alkaloids as anti-inflammatory treatments for IBD. The study demonstrated that nicotine significantly reduced colitis symptoms, including inflammation and tissue damage, while anatabine showed similar beneficial trends.
This work underscores a key advantage of our model: nicotine does not exhibit anti-inflammatory effects in conventional murine immune systems, a limitation that often reduces the translational value of standard mouse models. By using mice reconstituted with a human immune system, our model more accurately reflects human immune responses, making it a more predictive and reliable platform for evaluating therapeutic efficacy in IBD.
The right mouse model for your research experimentation
-
CD34+ Humanized Immune System Mouse Model
Evaluate both the efficacy and immunotoxicity of your lead candidate using a fully reconstituted human immune system, generated by engrafting CD34+ hematopoietic stem cells into highly immunodeficient mice.
See the mouse model -
Conventional Mouse Model
Run robust and cost-effective preclinical studies in C57BL/6 or BALB/c mice. Ideal for early-stage research in oncology and inflammation offering reliable results within a functional murine immune system.
See the mouse model
Contact us to have more information about a mouse model
Contact usKnow Everything About the IBD models in Humanized Immune System Mouse Model
-
What are the human immune cells contributing to the IBD onset ?
Following DSS exposure, we observed a marked infiltration of human T cells and macrophages in the inflamed colonic tissue, highlighting a robust human immune response. Additionally, there was a notable upregulation of regulatory T cells (Tregs) in peripheral blood, reflecting systemic immune modulation and further validating the model’s translational relevance. -
How many mice per arm do you recommend ?
Conventional IBD mouse models are known for their inherent variability, and our humanized mouse model is no exception. To ensure robust and interpretable results, we recommend using a minimum of 8 animals, housed across 2 cages, to account for biological variation while maintaining statistical reliability. -
Do you work with validated batches of DSS ?
Yes, we consistently validate each new batch of DSS to ensure that the selected concentration reliably produces the expected level of disease severity, maintaining consistency and reproducibility across studies. -
Do you recommend to boost the differentiation of myeloid cells ?
Yes, Unless the study focuses exclusively on the role of T cells, it is optimal to enhance monocyte and macrophage representation in the system to more closely mimic the human immune response and better reflect the complexity of IBD pathophysiology. -
Do you also provide IBD models in conventional mouse models ?
Yes, absolutely. We can induce IBD in immunocompetent mouse models and apply our full range of expertise and analytical readouts to investigate the contribution of the native murine immune system, providing valuable insights into disease mechanisms and treatment effects in a functional mouse immune context.
You have more questions ?
If you have further questions or would like to discuss how our IBD model can be tailored to your specific research needs, our scientific team is here to help. With over a decade of experience in both CD34+ humanized models and IBD research, we’re committed to providing clear, responsive support and working closely with you to design studies that align with your objectives. Don’t hesitate to reach out - we’re always happy to share our expertise and explore solutions together.
Contact us