Inflammatory Disease: Pulmonary Inflammation and Fibrosis
Lung Inflammation in Human Immune System Mouse Model
Contact usLung inflammation and fibrosis, including conditions such as idiopathic pulmonary fibrosis (IPF), is a progressive and often fatal disease characterized by chronic inflammation, immune dysregulation, and excessive deposition of extracellular matrix in the lung, leading to impaired respiratory function. Understanding the interplay between the immune system and fibrotic processes is critical for developing effective therapies.
TransCure bioServices offers a lung inflammation/fibrosis models in humanized mice, induced by either bleomycin or ovalbumin (OVA) challenge. These models trigger inflammatory and fibrotic responses in the lung. In humanized mice, these responses occur in the context of a fully functional human immune system, including T cells, macrophages, dendritic cells, and monocytes, enabling human-relevant assessment of drug efficacy and immune involvement.
Main characteristics
-
Pulmonary Fibrosis
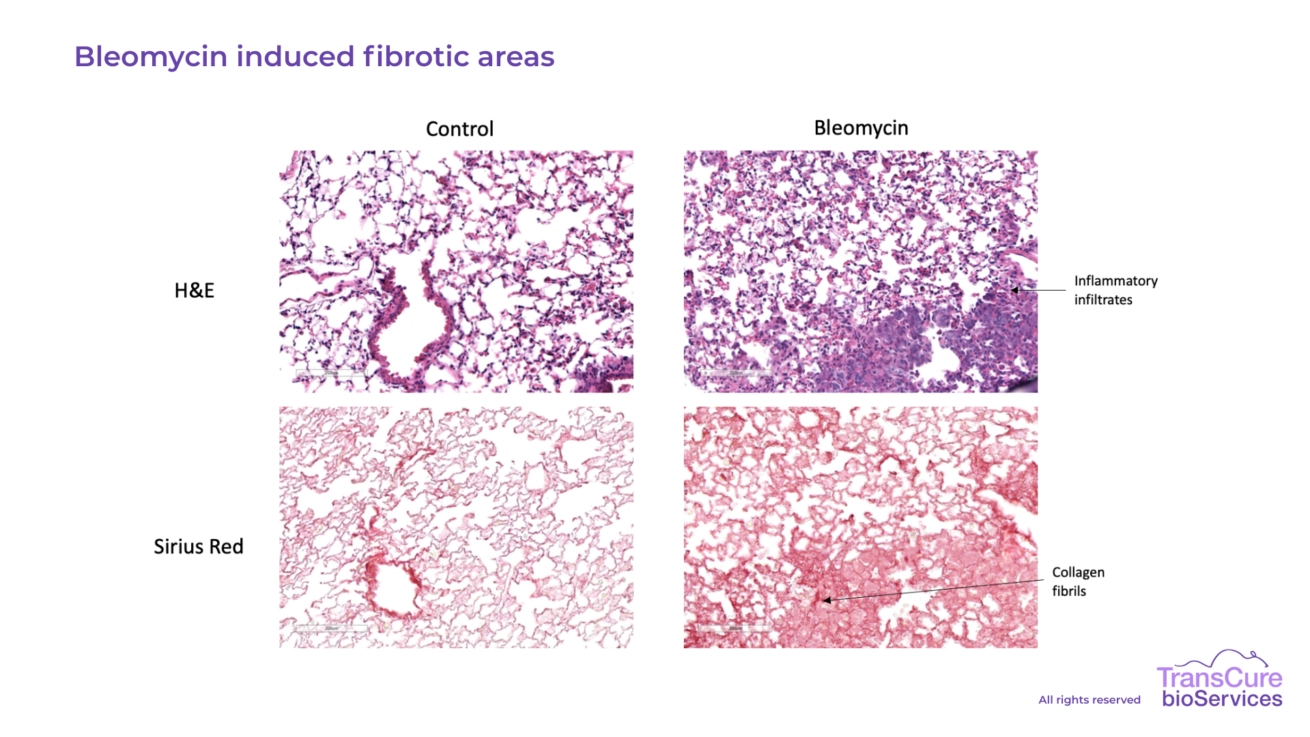
Lung fibrosis is induced by intra-tracheal administration of bleomycin, triggering localized injury and inflammation. By two weeks post-administration, the lungs exhibit both fibrotic remodeling and significant infiltration of human immune cells, including macrophages, T cells, and monocytes. This reflects the human-like immune involvement in fibrotic progression, making the model highly relevant for preclinical evaluation of anti-fibrotic and immunomodulatory therapies.
-
Ovalbumin Challenge
In the ovalbumin (OVA) challenge model, humanized mice are sensitized via intraperitoneal immunization with OVA for two weeks, followed by intra-nasal OVA administration to induce a robust allergic airway response. This challenge triggers a typical asthma-like reaction, characterized by infiltration of human immune cells—including eosinophils, T cells, and dendritic cells—into the lung tissue and bronchoalveolar lavage (BAL) fluid, providing a clinically relevant platform for studying human immune mechanisms in allergic airway inflammation.
-
Lung and BALF Immunophenotyping
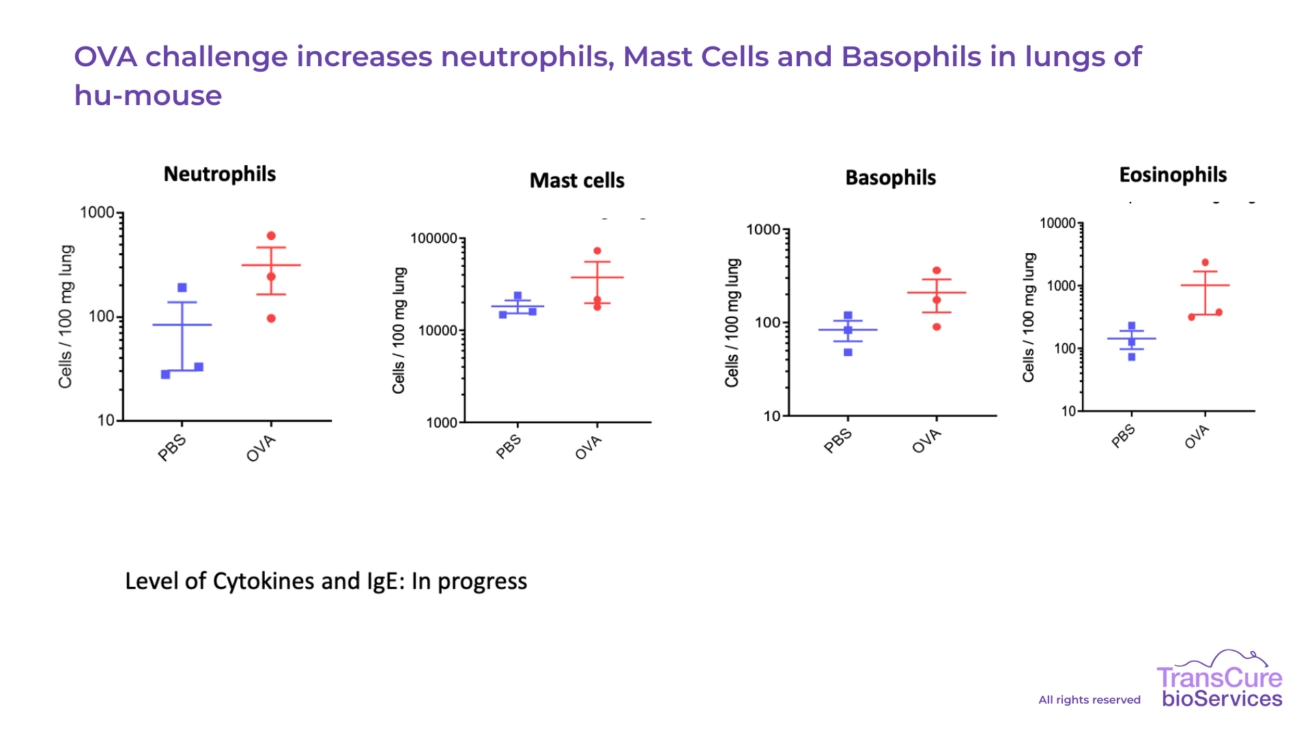
Our team has developed robust capabilities to characterize human immune cell populations in both the lung tissue and bronchoalveolar lavage fluid (BALF) of CD34+ humanized mice. Using advanced flow cytometry and immunohistochemistry, we have identified the presence of key human immune subsets, including macrophages, T cells, mast cells, basophils, neutrophils, and eosinophils. These readouts provide valuable insights into immune cell recruitment and activation in pulmonary inflammation and fibrosis models, supporting detailed and translational analysis of therapeutic responses.
-
Fibrosis Quantification
Lung fibrosis is quantified through Picosirius Red staining, which highlights collagen deposition and enables precise measurement of fibrotic severity. In parallel, H&E staining is used to assess tissue architecture and immune cell infiltration, providing a comprehensive overview of both fibrotic remodeling and inflammatory response. These histological analyses support high-resolution evaluation of treatment efficacy in lung fibrosis models.
-
Clinical Scoring
Humanized mice are closely monitored throughout the study to assess a composite clinical score, which includes indicators such as reduced activity, shortness of breath, and lack of responsiveness. Additionally, body weight is tracked regularly as a general marker of health and disease progression. At the study endpoint, lungs are collected and weighed, providing a quantitative measure of inflammation and tissue remodeling, and serving as a complementary readout to histological and cellular analyses.
Applications
The right mouse model for your research experimentation
-
CD34+ Humanized Immune System Mouse Model
Evaluate both the efficacy and immunotoxicity of your lead candidate using a fully reconstituted human immune system, generated by engrafting CD34+ hematopoietic stem cells into highly immunodeficient mice.
See the mouse model -
Conventional Mouse Model
Run robust and cost-effective preclinical studies in C57BL/6 or BALB/c mice. Ideal for early-stage research in oncology and inflammation offering reliable results within a functional murine immune system.
See the mouse model
Contact us to have more information about a mouse model
Contact usKnow everything about this model
-
How do you ensure sufficient myeloid cell representation in the model?
To achieve a robust and physiologically relevant presence of myeloid cells—including monocytes, macrophages, dendritic cells, and granulocytes—we apply an optimized boosting strategy. This involves hydrodynamic injection of plasmids encoding human cytokines such as IL-3, IL-4, IL-15, GM-CSF, and FLT3L, which selectively enhance myeloid and innate immune cell differentiation without overstimulating the T cell compartment. This approach ensures a balanced and functional human immune system, well-suited for studies involving lung inflammation.
You have more questions ?
If you have further questions or would like to discuss how our lung inflammation models can be tailored to your specific research needs, our scientific team is here to help. We’re committed to providing clear, responsive support and working closely with you to design studies that align with your objectives. Don’t hesitate to reach out - we’re always happy to share our expertise and explore solutions together
Contact us